Published online Feb 21, 2013. doi: 10.3748/wjg.v19.i7.1020
Revised: November 19, 2012
Accepted: December 5, 2012
Published online: February 21, 2013
Processing time: 155 Days and 17.8 Hours
To summarize the evidence about the association between red and processed meat intake and the risk of esophageal cancer, we systematically searched the PubMed and EMBASE databases up to May 2012, with a restriction to English publications, and the references of the retrieved articles. We combined the study-specific relative risks (RRs) and 95%CI, comparing the highest with the lowest categories of consumption by using a random-effects model. A total of 4 cohort studies and 23 case-control studies were included in the meta-analysis. The combined RRs (95%CI) of the cohort studies comparing the highest and lowest categories were 1.26 (1.00-1.59) for red meat and 1.25 (0.83-1.86) for processed meat. For the case-control studies, the combined RRs (95%CI) comparing the highest and lowest categories were 1.44 (1.16-1.80) for red meat and 1.36 (1.07-1.74) for processed meat. Findings from this meta-analysis suggest that a higher consumption of red meat was associated with a greater risk of esophageal cancer.
- Citation: Choi Y, Song S, Song Y, Lee JE. Consumption of red and processed meat and esophageal cancer risk: Meta-analysis. World J Gastroenterol 2013; 19(7): 1020-1029
- URL: https://www.wjgnet.com/1007-9327/full/v19/i7/1020.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i7.1020
The incidence rate of esophageal cancer ranked eighth worldwide, accounting for 3.8% of all new cancers, and its mortality rate ranked sixth, accounting for 5.4% of all cancer deaths in 2008[1]. The most predominant histological types of esophageal cancer are esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC), representing distinct characteristics in patterns of cancer development and risk factors[2].
Given that mutagenic compounds such as heterocyclic amines (HCAs), polycyclic aromatic hydrocarbons (PAHs), and N-nitroso compounds (NOCs) generated from red and processed meats were associated with cancer development[3], concerns about a high incidence of esophageal cancer related to a high consumption of red and processed meats have been increasing. In 2007, a consensus report of experts assembled by the World Cancer Research Fund (WCRF) and the American Institute for Cancer Research (AICR)[4] concluded through review of studies published up to 2004 that there were suggestive but inconclusive associations between red and processed meat consumption and esophageal cancer risk. The WCRF/AICR expert report also indicated that the lack of consistent results may be because of insufficient data, especially from prospective cohort studies. Another review of studies published up to 2005[5] suggested a possible increased risk of esophageal cancer with processed meat (9 case-control studies) and combined white and red meat (2 cohort and 18 case-control studies); however, this study concluded that more prospective data involving a larger number of cases would be needed to determine the association between meat consumption and the risk of esophageal cancer.
Since the completion of the two reviews, the results of the large prospective studies as well as new or updated case-control studies that examined association between red and processed meats and esophageal cancer risk have been published, but no meta-analysis of the prospective cohort studies has been reported. We, therefore, performed a meta-analysis of large prospective cohort and case-control studies to summarize the association between red and processed meat intake and the risk of esophageal cancer. We also quantified the dose-response relationships in the analysis of the cohort studies.
Two authors (Choi Y, Song S) independently performed a systematic search of published articles using the PubMed and EMBASE databases up to May 2012. We used the following search terms: “oesophageal or esophageal or esophagus or oesophagus” and “cancer or neoplasm or carcinoma” and “cohort or prospective or case-control” and “food or diet or meat”. We also reviewed the reference lists from the retrieved articles and those from previous review studies to identify additional relevant studies that may not have been identified by our database searches.
Studies were included in our meta-analysis if they met the following criteria: (1) either a cohort or case-control design was used; (2) relative risk (RR) estimates and the 95%CI were provided for the association between red and/or processed meat intake and esophageal cancer; (3) the outcomes of interest were either the overall incidence of esophageal cancer or the two main histological subtypes, ESCC or EAC; and (4) the study was published in English. We included studies that reported the associations of esophageal cancer with exposures identified as “red meat” or “processed meat” and individual food items within the two groups. Studies generally included beef, pork, minced meat, lamb, veal, and offal (e.g., liver, kidney) for unprocessed red meat and sausage, ham, bacon, salami, luncheon meat, or frankfurters, and any types of meat that were processed by smoking, curing, salting, or the addition of preservatives for processed meat. We excluded studies providing no apparent classification of meat or studies reporting a combination of red and white meat (e.g., poultry). If data were duplicated in more than 1 study, the latest studies were included.
We independently extracted the following data from each study, according to the meta-analysis of observational studies in epidemiology guidelines[6], and any discrepancies were resolved by discussion: the first author’s last name, the publication year, the country where the study was conducted, the study period, the age range of the subjects, the number of cases and controls or the cohort size, the measures and comparison levels of the exposures, the multivariate adjusted RRs with corresponding 95%CI for the highest vs lowest categories of red or processed meat intake, and the variables that were adjusted for in the analysis. For each study, we used the most fully adjusted RRs in the multivariate model. Any disagreements were resolved through consensus. The same two authors assessed the quality of the studies based on the Newcastle-Ottawa Scale, which ranged from 1 to 9 stars[7]. The average score for each study was used in the analysis.
We conducted separate meta-analyses for case-control and cohort studies, using results that compared red and processed meat intake as well as those that assessed each type individually. We also performed a meta-analysis combining both case-control and cohort studies. Using a random-effects model that considered both within and between study variation[8], we combined the study-specific multivariate RRs and 95%CI, comparing the highest and the lowest categories of red and processed meat intake.
We assessed the statistical heterogeneity among the studies by using Q and I2 statistics[9], where significance was reached at P < 0.1. Publication bias was evaluated by using the Egger asymmetry test[10], with significant level at P < 0.05. We investigated the potential sources of heterogeneity among the studies by conducting subgroup and meta-regression analyses for histological subtype (ESCC and EAC), sex (males, females, and both sexes), study location (Asia, Europe, North America, and South America), study quality, and confounders adjusted for in the analysis [alcohol, smoking, body mass index (BMI), and fruit and/or vegetable]. We also conducted the sensitivity analysis for case-control and cohort studies separately, omitting each study individually to evaluate whether the results could have been affected substantially by any one study.
In a sensitivity analysis, we estimated a dose-response for combined RRs for 100 g/d increments of red or processed meats for 3 cohort studies[11-13], which are less prone to selection or recall bias than case-control studies. We did not include one study (Yu et al[14]), that presented binary categories of exposure for a dose-response analysis. For two studies[11,13], the estimates were rescaled into 100 g/d increments. All statistical analyses were performed with Stata software, version 11 (Stata Corp., College Station, TX, United States). P < 0.05 considered statistically significant.
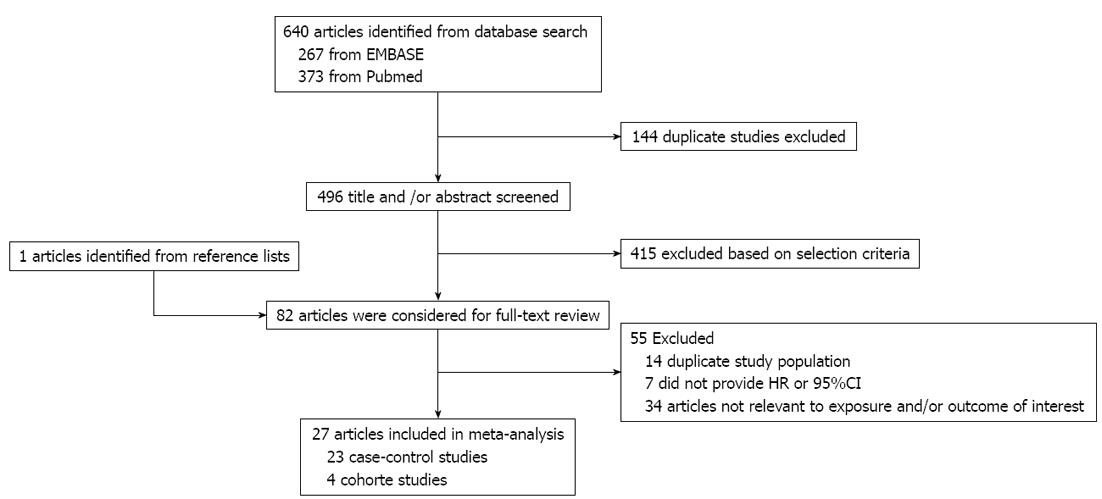
The preliminary literature search yielded 640 articles. Of these, 81 articles and 1 additional article identified from the reference lists were considered for further review (Figure 1). After the full-text review, 7 articles that did not provide RRs or 95%CI, 14 articles that used duplicated study populations, and 34 articles that were unrelated to exposure or outcomes of interest were excluded. A total of 27 articles were included in the meta-analysis; 22 articles (4 cohort and 18 case-control studies) that reported findings on red meat and 18 articles (3 cohorts and 15 case-controls) that reported findings on processed meat were included in the meta-analysis.
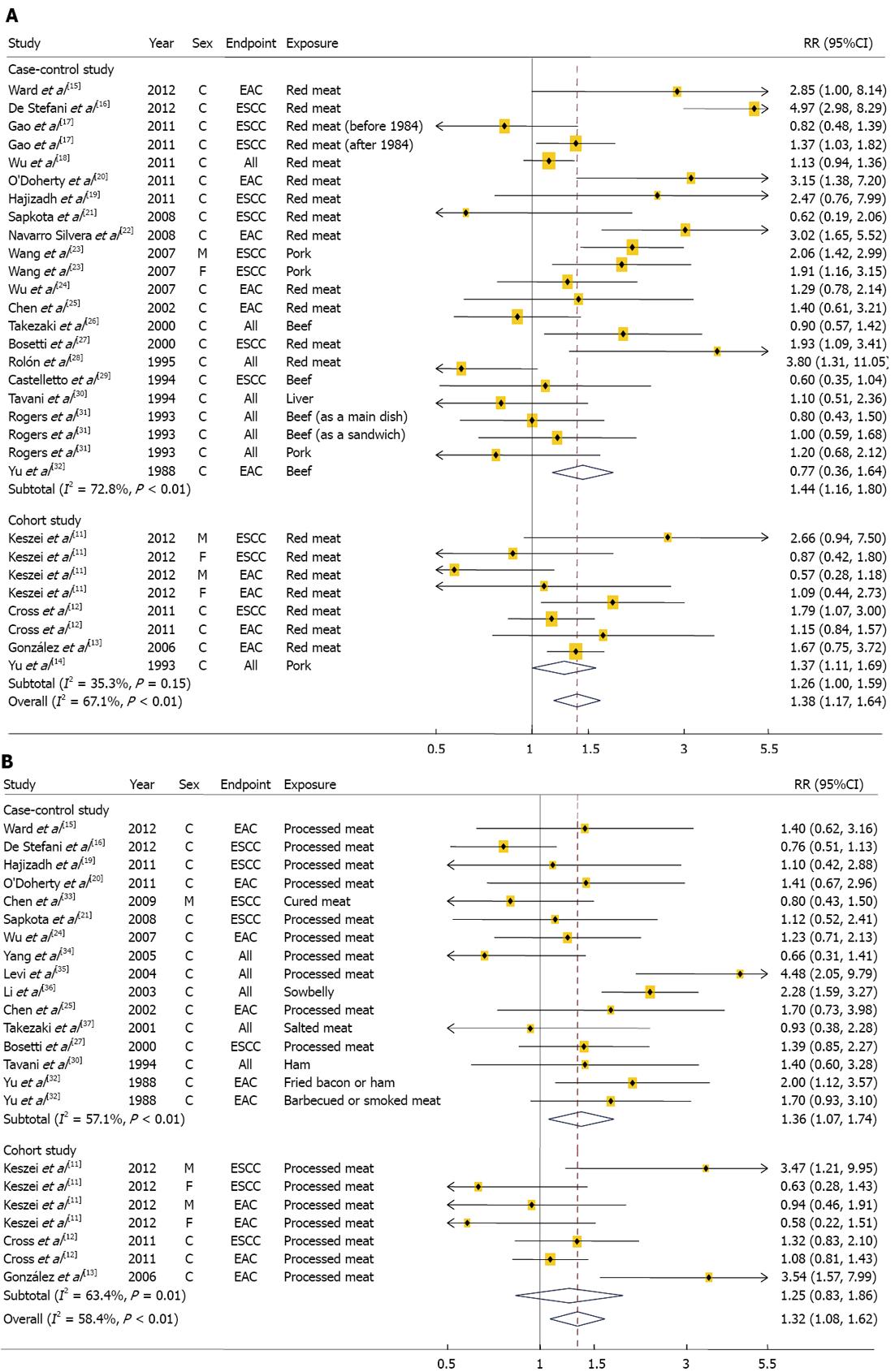
We identified 4 cohorts studies[11-14] involving 2324 cases and 1 149 981 participants and 18 case-control studies[15-32] involving 5165 cases and 26 350 control subjects (Table 1). Two of the 22 studies reported results for both ESCC and EAC, 16 studies reported the results for either EAC or ESCC, and 6 reported results for overall esophageal cancer without the histological subtypes. Six studies were conducted in Asia, 6 in Europe, 7 in United States, and 3 in South America. The studies used either a food frequency questionnaire (FFQ) or a structured questionnaire form to measure red meat intake. Fifteen studies provided RR estimates that were adjusted for alcohol intake, 16 for smoking habit, 12 for BMI, and 7 for fruit and/or vegetable intake. Eight studies were given a score of 7 stars or above, representing a high quality of studies[7]. The combined RRs (95%CI) comparing the highest and lowest categories of red meat intake were 1.26 (1.00-1.59) for the 4 cohort studies and 1.44 (1.16-1.80) for the 18 case-control studies (Figure 2A). There was no evidence of heterogeneity among the cohort studies (P = 0.15, I2 = 35.3%), but there was a heterogeneity among the case-control studies (P < 0.01, I2 = 72.8%). Combining the two types of study design resulted in an overall combined RR of 1.38 (95%CI: 1.17-1.64; P for heterogeneity: P < 0.01, I2 = 67.1%). Excluding a single study did not substantially influence the combined estimates of the cohort or case-control studies. There was no statistical evidence of publication bias according to the Egger asymmetry test (P = 0.79 for cohort studies and P = 0.34 for case-control studies). Dose-response associations were examined in 3[11-13] of 4 cohort studies, showing the combined RRs of 1.05 (95%CI: 0.91-1.21; P for heterogeneity = 0.42, I2 = 0.2%) for every 100 g/d increment of red meat intake. The associations did not vary significantly by histological subtypes, study location, sex, and study quality (Table 2). In addition, the associations did not differ by adjusted confounding factors including alcohol, smoking, BMI, and fruit and vegetable intakes (data not shown).
| Ref. | Study period | Sex | No. of cases | No. cohorts or controls | Dietary assessment | Exposure and comparison level | Adjusted RR (95%CI) | Study quality1 | Adjustment for confounders |
| Cohort studies | |||||||||
| Keszei et al[11] | 1986-2002 | M | ESCC: 107 | 120 852 | FFQ 150 items | Red meat | 9 | Age, smoking (including years and numbers per day), total energy, BMI, alcohol drinking, vegetable, fruit, education, non-occupational PA | |
| F | EAC: 145 | ESCC | |||||||
| M | Q5 vs Q1 | 2.66 (0.94-7.48) | |||||||
| F | T3 vs T1 | 0.87 (0.42-1.79) | |||||||
| EAC | |||||||||
| M | Q5 vs Q1 | 0.57 (0.28-1.19) | |||||||
| F | T3 vs T1 | 1.09 (0.44-2.75) | |||||||
| Processed meat | |||||||||
| ESCC | |||||||||
| M | Q5 vs Q1 | 3.47 (1.21-9.94) | |||||||
| F | T3 vs T1 | 0.63 (0.28-1.44) | |||||||
| EAC | |||||||||
| M | Q5 vs Q1 | 0.94 (0.46-1.89) | |||||||
| F | T3 vs T1 | 0.58 (0.22-1.50) | |||||||
| Cross et al[12] | 1995-2006 | C | ESCC: 215 | 494 979 | FFQ 124 items | Red meat (Q5 vs Q1) | 9 | Age, sex, BMI, education, ethnicity, smoking, alcohol drinking, PA at work, vigorous PA, daily intakes of fruit, vegetable, saturated fat, energy | |
| EAC: 630 | ESCC | 1.79 (1.07-3.01) | |||||||
| EAC | 1.15 (0.84-1.57) | ||||||||
| Processed meat (Q5 vs Q1) | |||||||||
| ESCC | 1.32 (0.83-2.10) | ||||||||
| EAC | 1.08 (0.81-1.43) | ||||||||
| González et al[13] | 1992-1998 | C | EAC: 65 | 521 457 | FFQ 88-266 items | Red meat (T3 vs T1) | 1.67 (0.75-3.72) | 8 | Sex, height, weight, education, smoking, smoking intensity, work and leisure PA, intakes of alcohol, energy, vegetable, citrus fruit, non-citrus fruit, types of meat intake were mutually adjusted |
| Processed meat (T3 vs T1) | 3.54 (1.57-7.99) | ||||||||
| Yu et al[14] | 1974-1989 | C | All: 1162 | 12 693 | Questionnaire 15 items | Pork (never vs regular/occasional) | 1.37 (1.11-1.68) | 7 | Age, sex |
| Case-control studies | |||||||||
| Ward et al[15] | 1988-1993 | C | EAC: 124 | 449 | Questionnaire 100 items | Red meat (> 157.2 g/d vs ≤ 73.8 g/d) | 2.85 (1.00-8.16) | 5 | Age, sex, race, vital status, year of birth, sex, No. of cigarettes per day, BMI, intakes of retinoic acid, folate, riboflavin, zinc, carbohydrate, protein, total energy. |
| Processed meat (> 52.3 g/d vs ≤ 16.1 g/d) | 1.40 (0.62-3.15) | ||||||||
| De Stefani et al[16] | 1996-2004 | C | ESCC: 234 | 2020 | FFQ 64 items | Red meat ( T3 vs T1) | 4.97 (2.98-8.29) | 7 | Age, sex, residence, education, BMI, smoking, drinking, mate temperature, total energy, total intakes of vegetable and fruit, scored pattern |
| Processed meat (T3 vs T1) | 0.76 (0.51-1.13) | ||||||||
| Gao et al[17] | 1997-2005 | C | ESCC: 600 | 1514 | Questionnaire 35 items | Red meat (> weekly vs monthly/seldom/never) | 1.37 (1.03-1.82) | 5 | Age, sex, geographic region |
| Wu et al[18] | 2003-2007 | C | All: 1495 | 3819 | FFQ | Red meat (Q4 vs Q1) | 1.13 (0.94-1.36) | 7 | Age, sex, education, previous income, BMI, pack-years smoking, weekly ethanol intake, study area |
| Hajizadeh et al[19] | N/A | C | ESCC: 47 | 96 | FFQ168 items | Red meat (T3 vs T1) | 2.47 (0.76-7.96) | 6 | Age, sex, education, tobacco smoking, symptomatic gastroesophageal reflux, BMI, total energy |
| Processed meat (T3 vs T1) | 1.10 (0.36-2.47) | ||||||||
| O'Doherty et al[20] | 2002-2005 | C | EAC: 221 | 256 | FFQ101 items | Red meat (Q4 vs Q1) | 3.15 (1.38-7.20) | 7 | Age, sex, smoking, BMI 5 yr before interview date, education, job type, Intakes of energy, fruit, vegetable, alcohol (g/d), Helicobacter pylori infection, nonsteroidal anti-inflammatory drug use 5 yr before, interview date, gastroesophageal reflux symptoms, location, types of meat intake were mutually adjusted |
| Processed meat (Q4 vs Q1) | 1.41 (0.67-2.95) | ||||||||
| Sapkota et al[21] | 1999-2003 | C | ESCC: 187 | 1110 | Questionnaire 23 items | Red meat (≥ 1/wk vs < 1/mo) | 0.62 (0.19-2.09) | 6 | Age, sex, country, tobacco pack-year, education, BMI, frequency of alcohol consumption, vegetable, fruit consumption |
| Processed meat (≥ 1 time/wk vs < 1 time/mo) | 1.12 (0.52-2.41) | ||||||||
| Navarro Silvera et al[22] | 1993-1995 | C | EAC: 282 | 687 | FFQ104 items | Red meat (high vs low) | 3.02 (1.65-5.52) | 7 | Age, sex, study site, race, proxy status, income, education, BMI, No. of smoking cigarettes per day, intakes of beer, wine, liquor, and energy |
| Wang et al[23] | 2004-2006 | M | ESCC: 355 | 408 | Questionnaire | Pork (often vs none/seldom) | 2.06 (1.42-2.99) | 5 | Age, sex, marital status, education |
| F | 1.91 (1.16-3.16) | ||||||||
| Wu et al[24] | 1992-1997 | C | EAC: 206 | 1308 | Questionnaire 124 items | Red meat (Q4 vs Q1) | 1.29 (0.8-2.2) | 5 | Age, sex, race, birthplace, education, smoking, BMI, reflux, use of vitamins, total energy |
| Processed meat (Q4 vs Q1) | 1.23 (0.7-2.1) | ||||||||
| Chen et al[25] | 1988-1993 | C | EAC: 124 | 449 | Questionnaire54 items | Red meat (Q4 vs Q1) | 1.4 (0.61-3.2) | 7 | Age, sex, energy intake, respondent type, BMI, alcohol drinking, smoking, education, family history, vitamin supplement use, age squared for EAC |
| Processed meat (Q4 vs Q1) | 1.7 (0.71-3.9) | ||||||||
| Takezaki et al[26] | 1988-1997 | M | All: 284 | 11 888 | Questionnaire | Beef (≥ 3/wk vs ≤ 3/mo) | 0.9 (0.6-1.5) | 5 | Age, year and season of visit, smoking, drinking |
| Bosetti et al[27] | 1992-1997 | C | ESCC:304 | 743 | FFQ78 items | Red meat (Q5 vs Q1) | 1.93 (1.09-3.41) | 5 | Age, sex, area of residence, education, tobacco smoking, alcohol drinking, non-alcohol energy |
| Rolón et al[28] | 1988-1991 | C | All: 131 | 379 | FFQ | Red meat (highest vs lowest) | 3.8 (1.3-11.0) | 5 | Age, sex, alcohol, smoking, design variable of the study, hospital group, intakes of red meats, fats, fish, milk |
| Castelletto et al[29] | 1986-1989 | C | ESCC: 131 | 261 | FFQ10 food groups | Beef (≥ daily vs < daily) | 0.6 (0.3-0.9) | 6 | Age, sex, design variable, hospital, education, No. of cigarettes smoking per day, intakes of alcohol, barbecued meat, potatoes, raw vegetables, cooked vegetables |
| Tavani et al[30] | 1984-1992 | C | All: 46 | 230 | FFQ14 items | Ham (Q3 vs Q1) | 1.4 (0.6-3.3) | 5.5 | Age, sex, education, total alcohol intake |
| Liver (Q2 vs Q1) | 1.1 (0.5-2.3) | ||||||||
| Rogers et al[31] | 1983-1987 | C | All: 127 | 466 | FFQ125 items | Beef (≥ 1/wk vs < 1/wk) | 5 | Age, sex, pack-years of cigarette, drink-years of alcohol, energy intake, beta-carotene intake, ascorbic acid intake | |
| As a main dish | 0.8 (0.4-1.4) | ||||||||
| As a sandwich | 1.0 (0.6-1.7) | ||||||||
| Pork (≥ 1/wk vs < 1/wk) | 1.2 (0.8-2.5) | ||||||||
| Yu et al[32] | 1975-1981 | C | Beef: 267 | Beef: 267 | Questionnaire10 food groups | Beef (≥ 5/wk vs ≤ 1/wk) | 1.3 (0.6-2.7) | 5 | Age, sex, race |
| Fried bacon or ham: 265 | Fried bacon or ham: 265 | Fried bacon or ham ( ≤ 1/wk vs≥ 5/wk ) | 2.0 (1.1-3.5) | ||||||
| Barbecued or smoked meat: 268 | Barbecued or smoked meat: 268 | Barbecued or smoked meat (≥ 2/wk vs ≤ 1/wk) | 1.7 (0.9-3.0) | ||||||
| Chen et al[33] | 1996-2005 | M | ESCC: 320 | 709 | Questionnaire6 items | Cured meat (≥ 1/wk vs < 1/wk ) | 0.8 (0.4-1.4) | 5 | Age, educational level, ethnicity, source of hospital, smoking, alcohol drinking, areca nut chewing |
| Yang et al[34] | 2003-2004 | C | All: 185 | 185 | Questionnaire 9 Items | Processed meat (> 3 meals/wk vs < 1 meal/wk) | 0.66 (0.31-1.41) | 5.5 | Family history of esophageal cancer, occupation, smoking, drinking, eating hot food, eating speed, intakes of vegetables, fruit, pickled vegetables, fresh meat, egg, tea, water supply |
| Levi et al[35] | 1992-2002 | C | All:138 | 660 | FFQ79 items | Processed meat(> 3.2 freq/wk vs < 0.8 freq/wk) | 4.48 (2.05-9.79) | 6 | Age, sex, education, smoking, intakes of alcohol, energy, fruit and vegetable intake |
| Li et al[36] | 1997-2000 | C | All:1248 | 1248 | Questionnaire 12 items | Sowbelly (daily vs < 1/wk) | 2.28 (1.6-3.3) | 5 | Age, sex, income, residence, occupation, alcohol, tobacco |
| Takezaki et al[37] | 1995-2000 | C | All: 199 | 333 | Questionnaire19 items | Salted meat (≥ 1/wk vs < 1/mo) | 0.93 (0.38-2.29) | 6 | Age, sex, smoking, drinking |
| Red meat | Processed meat | |||||||
| Factors | Studies (n) | Ref. | RR (95%CI) | P for heterogeneity | Studies (n) | Ref. | RR (95%CI) | P for heterogeneity |
| Histological subtypes | ||||||||
| EAC | 9 | [11-13,15,20,22,24,25,32] | 1.42 (1.02-1.98) | 0.19 | 8 | [11-13,15,20,24,25,32] | 1.38 (1.07-1.78) | 0.3 |
| ESCC | 9 | [11,12,16,17,19,21,23,27,29] | 1.55 (1.10-2.17) | 7 | [11,12,16,19,21,27,33] | 1.08 (0.80-1.44) | ||
| Study location | ||||||||
| Asia | 6 | [14,17,18,19,23,26] | 1.33 (1.09-1.62) | 0.67 | 5 | [19,33,34,36,37] | 1.09 (0.61-1.95) | 0.65 |
| Europe | 6 | [11,13,20,21,27,30] | 1.33 (0.86-2.07) | 7 | [11,13,20,21,27,30,35] | 1.49 (0.99-2.23) | ||
| United States | 7 | [12,15,22,24,25,31,32] | 1.32 (1.03-1.70) | 5 | [12,15,21,25,32] | 1.30 (1.08-1.57) | ||
| South America | 3 | [16,28,29] | 2.20 (0.48-10.04) | 1 | [16] | 0.76 (0.51-1.13) | ||
| Sex | ||||||||
| Male | 3 | [11,23,26] | 1.26 (0.66-2.41) | 0.88 | 2 | [11,33] | 1.24 (0.58-2.65) | 0.14 |
| Female | 2 | [11,23] | 1.31 (0.78-2.21) | 1 | [11] | 0.61 (0.33-1.13) | ||
| Both | 19 | [12-22,24,25,27-32] | 1.42 (1.17-1.71) | 16 | [12,13,15,16,19-21,24,25,27,30,32,34-37] | 1.43 (1.15-1.77) | ||
| Study quality1 | ||||||||
| ≥ 7 | 8 | [11-14,16,18,20,22] | 1.60 (1.20-2.13) | 0.23 | 6 | [11-13,16,20,25] | 1.20 (0.88-1.62) | 0.42 |
| < 7 | 14 | [15,17,19,21,23-32] | 1.25 (1.02-1.54) | 12 | [15,19,21,24,27,30,32-37] | 1.43 (1.11-1.86) | ||
We conducted a meta-analysis of 3 cohort studies[11-13], which included 1162 cases and 1 137 288 participants and 15 case-control studies[15,16,19-21,24,25,27,30,32-37], which included 3851 cases and 10 064 controls (Table 1). Two of the 18 studies examined both ESCC and EAC as the primary endpoints, 13 studies reported the results for either EAC or ESCC and 5 did not differentiate between histological subtypes. Five studies were conducted in Asia, 7 in Europe, 5 in United States, and 1 in South America. The studies used either a FFQ or a structured questionnaire form to measure processed meat intake. Fourteen studies provided RR estimates that were adjusted for alcohol intake, 15 for smoking habit, 10 for BMI, and 8 for fruit and/or vegetable intake. Six studies were given a score of 7 or greater, indicating a high methodological quality[7].
In a meta-analysis of the 15 case-control studies, we found that the highest categories of processed meat intake were associated with a 36% increase in esophageal cancer risk when compared with the lowest categories (95%CI: 1.07-1.74; Figure 2B); however, we found a non-significant, positive association when we examined only the cohort studies (RR: 1.25; 95%CI: 0.83-1.86). When we examined whether an individual study was the source of heterogeneity among either the cohort or case-control studies, there were heterogeneities between the case-control studies (P < 0.01, I2 = 57.1%) and the cohort studies (P = 0.01, I2 = 63.4%). When the results from the cohort and case-control studies were combined, the overall combined RR comparing the highest and the lowest category of processed meat was 1.32 (95%CI: 1.08-1.62; P for heterogeneity: P < 0.01, I2 = 58.4%). The heterogeneity observed between the prospective studies of processed meat intake and esophageal cancer risk was no longer significant (P = 0.12) after excluding a study by González et al[13]. However, excluding any one case-control study from the analysis did not influence the heterogeneity findings observed among case-control studies.
No publication bias was found for either the cohort or case-control studies (P = 0.65 for the cohort studies and P = 0.80 for the case-control studies). In a dose-response meta-analysis of 3 cohort studies, we found that each 100 g/d increase in processed meat intake was positively, but not significantly, associated with esophageal cancer risk (RR: 1.37; 95%CI: 0.88-2.13). There was no evidence of heterogeneity (P = 0.17, I2 = 33.5%).
When stratifying the analyses by histological subtypes, study location, sex, and study quality, we found no significant differences in the associations, although the magnitude of the associations differed slightly in these subgroups (Table 2). The associations also did not vary by adjusted confounding factors including alcohol, smoking, BMI, and fruit and vegetable intakes (data not shown).
To our knowledge, this is the first systematic meta-analysis of cohort and case-control studies to summarize the evidence regarding the association between red or processed meat intake and the risk of esophageal cancer. High red meat consumption was associated with a 38% higher risk of esophageal cancer compared to low consumption in a meta-analysis of both case-control and cohort studies. A 26% higher risk of esophageal cancer was observed among those who had high red meat intake compared to those with low intake in a meta-analysis of 4 cohort studies. With regard to processed meat, we found a higher risk of esophageal cancer with high processed meat intake compared to low intake in a meta-analysis of case-control studies, but the combined estimate of cohort studies did not reach statistical significance. Prospective cohort studies are less prone to selection or recall bias compared to case-control studies, which is critical in research of diet and cancer etiology. Therefore, a significant association in only the case-control studies and not in the meta-analysis of the 3 cohort studies could not provide adequate supportive evidence of an increased risk associated with processed meat consumption. However, the results for more prospective cohort studies need to be reported to obtain a clearer conclusion.
There are possible underlying mechanisms linking the consumption of red and processed meats and the incidence of cancer. HCAs and PAHs are chemical compounds with mutagenic potential that are formed when meat is boiled, fried, or grilled at high temperatures[3]. Animal studies have suggested that these two mutagenic compounds may induce changes in DNA, possibly promoting carcinogenesis[3,38]. Another class of meat-related mutagen is NOCs, the majority of which are potent carcinogens[39] formed either endogenously or exogenously. Processed meat is typically preserved by adding nitrate or nitrite, which increases the formation of NOCs[3]. Heme iron, largely derived from red meat sources, has been suggested to promote the endogenous formation of NOCs[40]. There is only limited epidemiological evidence, however, to suggest that the dietary intake of nitrite or nitrosamine is positively associated with the risk of esophageal cancer[5]. The esophagus is frequently exposed to these dietary mutagenic and/or carcinogenic compounds as stomach and colon, permitting food to pass from the esophagus into the stomach. While the specific mechanism by which meat causes esophageal cancer has not been fully elucidated, one likely reason may involve the potential for increase the susceptibility to carcinogenesis by repeated exposure of esophagus to the mutagenic and/or carcinogenic compounds, given their effects on carcinogenesis in animal models[3,38,39].
The results from the subgroup and meta-regression analysis could not completely explain the potential sources of between-study heterogeneity because we did not observe statistically significant differences by histological subtype, study location, sex, or study quality. For red meat intake, it appeared that a single study did not substantially influence the overall combined RR, whereas, the observed heterogeneity among the prospective studies of processed meat intake and esophageal cancer risk disappeared when the study by González et al[13] was excluded. However, the observed heterogeneity among the case-control studies of processed meat intake and esophageal cancer risk was not martially altered in sensitivity analyses excluding one study at a time.
Our meta-analysis had some limitations. Although the majority of the studies adjusted for known potential confounding factors, there may be a possibility that unidentified or residual confounding factors remained that were not adjusted for in the multivariate analysis or by covariates inadequately measured. Most studies, however, adjusted for alcohol and smoking, both of which are established risk factors for esophageal cancer. Additionally, we found an increased risk of esophageal cancer with high red meat intake in a meta-analysis of well-scored studies, which were relatively recent and adjusted for various potential confounding factors. The random measurement error of meat consumption that occurred during dietary assessment or the systematic error resulting from recall or selection bias in the case-control studies may have influenced our findings; however, we found a statistically significant association between red meat intake and esophageal cancer risk in a meta-analysis of prospective studies, which supports the hypothesis that red meat intake increases the risk of esophageal cancer.
Our meta-analysis also included several strengths. Our meta-analysis updated the recent large prospective and case-control studies with a larger number of cases that were not included in previous reviews. In particular, the inclusion of new data from large cohort studies, which were unavailable when earlier conclusions of these associations were made by the WCRF/AICR expert panel[4] or by a review study[5], enabled us to provide more unbiased evidence compared to the review that included only case-control studies. The findings from this meta-analysis were not subject to publication bias, indicating that the probability of publishing a study did not rely on the strength and direction of the associations.
The findings from our meta-analysis of either prospective cohort or case-control studies suggest that a high consumption of red meat may increase the risk of esophageal cancer. Although we found an increased risk in a meta-analysis of the case-control studies for processed meat intake in relation to esophageal cancer risk, the prospective cohort studies did not strongly support this evidence. There is a need for further large scale prospective studies to determine whether processed meat intake increases the risk of esophageal cancer. Moreover, further studies evaluating the effect of red or processed meat intake on individual histological subtypes of esophageal cancer are warranted.
We thank Dr. Cross for data provision for our dose-response meta-analysis part.
P- Reviewer Marjanovic G S- Editor Song XX L- Editor A E- Editor Xiong L
| 1. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11128] [Cited by in F6Publishing: 11775] [Article Influence: 841.1] [Reference Citation Analysis (4)] |
| 2. | Kamangar F, Chow WH, Abnet CC, Dawsey SM. Environmental causes of esophageal cancer. Gastroenterol Clin North Am. 2009;38:27-57, vii. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 269] [Cited by in F6Publishing: 287] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 3. | Cross AJ, Sinha R. Meat-related mutagens/carcinogens in the etiology of colorectal cancer. Environ Mol Mutagen. 2004;44:44-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 296] [Cited by in F6Publishing: 281] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 4. | World Cancer Research Fund / American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington DC: AICR 2007; . [Cited in This Article: ] |
| 5. | Jakszyn P, Gonzalez CA. Nitrosamine and related food intake and gastric and oesophageal cancer risk: a systematic review of the epidemiological evidence. World J Gastroenterol. 2006;12:4296-4303. [PubMed] [Cited in This Article: ] |
| 6. | Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008-2012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14425] [Cited by in F6Publishing: 16359] [Article Influence: 654.4] [Reference Citation Analysis (0)] |
| 7. | Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis. UK; University of Liverpool. UK: University of Liverpool 2012; . [Cited in This Article: ] |
| 8. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26739] [Cited by in F6Publishing: 29676] [Article Influence: 760.9] [Reference Citation Analysis (0)] |
| 9. | Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21630] [Cited by in F6Publishing: 24739] [Article Influence: 1075.6] [Reference Citation Analysis (0)] |
| 10. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34245] [Cited by in F6Publishing: 38800] [Article Influence: 1385.7] [Reference Citation Analysis (2)] |
| 11. | Keszei AP, Schouten LJ, Goldbohm RA, van den Brandt PA. Red and processed meat consumption and the risk of esophageal and gastric cancer subtypes in The Netherlands Cohort Study. Ann Oncol. 2012;23:2319-2326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Cross AJ, Freedman ND, Ren J, Ward MH, Hollenbeck AR, Schatzkin A, Sinha R, Abnet CC. Meat consumption and risk of esophageal and gastric cancer in a large prospective study. Am J Gastroenterol. 2011;106:432-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 13. | González CA, Jakszyn P, Pera G, Agudo A, Bingham S, Palli D, Ferrari P, Boeing H, del Giudice G, Plebani M. Meat intake and risk of stomach and esophageal adenocarcinoma within the European Prospective Investigation Into Cancer and Nutrition (EPIC). J Natl Cancer Inst. 2006;98:345-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 217] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 14. | Yu Y, Taylor PR, Li JY, Dawsey SM, Wang GQ, Guo WD, Wang W, Liu BQ, Blot WJ, Shen Q. Retrospective cohort study of risk-factors for esophageal cancer in Linxian, People’s Republic of China. Cancer Causes Control. 1993;4:195-202. [PubMed] [Cited in This Article: ] |
| 15. | Ward MH, Cross AJ, Abnet CC, Sinha R, Markin RS, Weisenburger DD. Heme iron from meat and risk of adenocarcinoma of the esophagus and stomach. Eur J Cancer Prev. 2012;21:134-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | De Stefani E, Deneo-Pellegrini H, Ronco AL, Boffetta P, Correa P, Aune D, Mendilaharsu M, Acosta G, Silva C, Landó G. Meat consumption, cooking methods, mutagens, and risk of squamous cell carcinoma of the esophagus: a case-control study in Uruguay. Nutr Cancer. 2012;64:294-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Gao Y, Hu N, Han XY, Ding T, Giffen C, Goldstein AM, Taylor PR. Risk factors for esophageal and gastric cancers in Shanxi Province, China: a case-control study. Cancer Epidemiol. 2011;35:e91-e99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Wu M, Zhang ZF, Kampman E, Zhou JY, Han RQ, Yang J, Zhang XF, Gu XP, Liu AM, van’t Veer P. Does family history of cancer modify the effects of lifestyle risk factors on esophageal cancer? A population-based case-control study in China. Int J Cancer. 2011;128:2147-2157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Hajizadeh B, Jessri M, Moasheri SM, Rad AH, Rashidkhani B. Fruits and vegetables consumption and esophageal squamous cell carcinoma: a case-control study. Nutr Cancer. 2011;63:707-713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | O’Doherty MG, Cantwell MM, Murray LJ, Anderson LA, Abnet CC. Dietary fat and meat intakes and risk of reflux esophagitis, Barrett’s esophagus and esophageal adenocarcinoma. Int J Cancer. 2011;129:1493-1502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Sapkota A, Hsu CC, Zaridze D, Shangina O, Szeszenia-Dabrowska N, Mates D, Fabiánová E, Rudnai P, Janout V, Holcatova I. Dietary risk factors for squamous cell carcinoma of the upper aerodigestive tract in central and eastern Europe. Cancer Causes Control. 2008;19:1161-1170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Navarro Silvera SA, Mayne ST, Risch H, Gammon MD, Vaghan TL, Chow WH, Dubrow R, Schoenberg JB, Stanford JL, West AB. Food group intake and risk of subtypes of esophageal and gastric cancer. Int J Cancer. 2008;123:852-860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Wang JM, Xu B, Rao JY, Shen HB, Xue HC, Jiang QW. Diet habits, alcohol drinking, tobacco smoking, green tea drinking, and the risk of esophageal squamous cell carcinoma in the Chinese population. Eur J Gastroenterol Hepatol. 2007;19:171-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Wu AH, Tseng CC, Hankin J, Bernstein L. Fiber intake and risk of adenocarcinomas of the esophagus and stomach. Cancer Causes Control. 2007;18:713-722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Chen H, Ward MH, Graubard BI, Heineman EF, Markin RM, Potischman NA, Russell RM, Weisenburger DD, Tucker KL. Dietary patterns and adenocarcinoma of the esophagus and distal stomach. Am J Clin Nutr. 2002;75:137-144. [PubMed] [Cited in This Article: ] |
| 26. | Takezaki T, Shinoda M, Hatooka S, Hasegawa Y, Nakamura S, Hirose K, Inoue M, Hamajima N, Kuroishi T, Matsuura H. Subsite-specific risk factors for hypopharyngeal and esophageal cancer (Japan). Cancer Causes Control. 2000;11:597-608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 27. | Bosetti C, La Vecchia C, Talamini R, Simonato L, Zambon P, Negri E, Trichopoulos D, Lagiou P, Bardini R, Franceschi S. Food groups and risk of squamous cell esophageal cancer in northern Italy. Int J Cancer. 2000;87:289-294. [PubMed] [Cited in This Article: ] |
| 28. | Rolón PA, Castellsagué X, Benz M, Muñoz N. Hot and cold mate drinking and esophageal cancer in Paraguay. Cancer Epidemiol Biomarkers Prev. 1995;4:595-605. [PubMed] [Cited in This Article: ] |
| 29. | Castelletto R, Castellsague X, Muñoz N, Iscovich J, Chopita N, Jmelnitsky A. Alcohol, tobacco, diet, mate drinking, and esophageal cancer in Argentina. Cancer Epidemiol Biomarkers Prev. 1994;3:557-564. [PubMed] [Cited in This Article: ] |
| 30. | Tavani A, Negri E, Franceschi S, La Vecchia C. Risk factors for esophageal cancer in lifelong nonsmokers. Cancer Epidemiol Biomarkers Prev. 1994;3:387-392. [PubMed] [Cited in This Article: ] |
| 31. | Rogers MA, Thomas DB, Davis S, Vaughan TL, Nevissi AE. A case-control study of element levels and cancer of the upper aerodigestive tract. Cancer Epidemiol Biomarkers Prev. 1993;2:305-312. [PubMed] [Cited in This Article: ] |
| 32. | Yu MC, Garabrant DH, Peters JM, Mack TM. Tobacco, alcohol, diet, occupation, and carcinoma of the esophagus. Cancer Res. 1988;48:3843-3848. [PubMed] [Cited in This Article: ] |
| 33. | Chen YK, Lee CH, Wu IC, Liu JS, Wu DC, Lee JM, Goan YG, Chou SH, Huang CT, Lee CY. Food intake and the occurrence of squamous cell carcinoma in different sections of the esophagus in Taiwanese men. Nutrition. 2009;25:753-761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Yang CX, Wang HY, Wang ZM, Du HZ, Tao DM, Mu XY, Chen HG, Lei Y, Matsuo K, Tajima K. Risk factors for esophageal cancer: a case-control study in South-western China. Asian Pac J Cancer Prev. 2005;6:48-53. [PubMed] [Cited in This Article: ] |
| 35. | Levi F, Pasche C, Lucchini F, Bosetti C, La Vecchia C. Processed meat and the risk of selected digestive tract and laryngeal neoplasms in Switzerland. Ann Oncol. 2004;15:346-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Li K, Yu P. Food groups and risk of esophageal cancer in Chaoshan region of China: a high-risk area of esophageal cancer. Cancer Invest. 2003;21:237-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Takezaki T, Gao CM, Wu JZ, Ding JH, Liu YT, Zhang Y, Li SP, Su P, Liu TK, Tajima K. Dietary protective and risk factors for esophageal and stomach cancers in a low-epidemic area for stomach cancer in Jiangsu Province, China: comparison with those in a high-epidemic area. Jpn J Cancer Res. 2001;92:1157-1165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 75] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 38. | Sugimura T, Wakabayashi K, Nakagama H, Nagao M. Heterocyclic amines: Mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci. 2004;95:290-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 513] [Cited by in F6Publishing: 449] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 39. | Mirvish SS. Role of N-nitroso compounds (NOC) and N-nitrosation in etiology of gastric, esophageal, nasopharyngeal and bladder cancer and contribution to cancer of known exposures to NOC. Cancer Lett. 1995;93:17-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 660] [Cited by in F6Publishing: 584] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 40. | Cross AJ, Pollock JR, Bingham SA. Haem, not protein or inorganic iron, is responsible for endogenous intestinal N-nitrosation arising from red meat. Cancer Res. 2003;63:2358-2360. [PubMed] [Cited in This Article: ] |