Published online Dec 28, 2013. doi: 10.3748/wjg.v19.i48.9351
Revised: September 27, 2013
Accepted: October 17, 2013
Published online: December 28, 2013
AIM: To assess retrospectively the epidemiological and clinical aspects of cystic echinococcosis (CE) and to evaluate follow-up and response to treatment in patients affected by CE.
METHODS: From January 2000 to December 2010, all patients affected by CE at the Infectious Diseases Units of the University of Catania and of Basilotta Hospital in Nicosia-Enna, were enrolled as participants in the study. Epidemiological, clinical and laboratory data were collected for each patient. Diagnosis of CE was performed using clinical imaging and laboratory parameters. Response to treatment was categorized as follows: “cure” as the disappearance or complete calcification of cyst/s; “improvement” as a reduction in the diameter and/or number of existing cysts; and “impairment” as an increase in the diameter and/or number of existing cyst/s and the onset of relapses (i.e., the onset of new cyst/s and an increase in the diameter of previously existing cyst/s and/or complications. Immunoglobulin E (IgE) titers and eosinophil percentages were evaluated at diagnosis, at six months after the initiation of treatment and again in the case of relapse. Hyper-eosinophilia was defined as an eosinophil percentage of ≥ 6%.
RESULTS: Thirty-two patients were diagnosed with CE in our Unit during the research period, with a male-female ratio of 2:1. At the time of diagnosis, 40% of patients presented a single CE cyst. Sixty percent showed multi-organ involvement. The liver-lung localization ratio was 2:1. Patients below the age of 50 at diagnosis were more likely to have multiple cysts (73.7% vs 35.5%, P < 0.05). Regarding treatment, 30 patients were treated medically and 16 surgically. Fourteen patients were treated both medically and surgically. Relapses were seen to be less frequent in patients treated with albendazole before and after surgery. Complete cure or an improvement was achieved in 23 patients. Impairment was observed in one patient. Two patients showed no improvement. Relapses were more frequent in those patients treated before 2005. At diagnosis, 71% of patients were positive for specific CE IgE, and 56.3% showed an eosinophil percentage of ≥ 6%. Patients who were diagnosed with hyper-eosinophilia developed complications more frequently than the other patients, but did not suffer relapses.
CONCLUSION: On the basis of our results, we propose cystic echinococcosis screening for family members of patients, appropriate pre- and post-surgery treatment and the assessment of anti-echinococcus IgE titer or eosinophil percentage as a therapy response marker in settings with limited resources.
Core tip: On the basis of the data presented, we suggest the use of specific immunoglobulin E detection and eosinophil percentage counts as therapeutic response markers, particularly in settings with limited resources. We also recommend: (1) routine screening for cystic echinococcosis in relatives (and/or close associates) of patients to facilitate the diagnosis of asymptomatic infection; (2) extension of the follow-up period after surgical and/or medical treatment for the early diagnosis of relapses; and (3) appropriate pre- and post-surgery therapy.
- Citation: Cappello E, Cacopardo B, Caltabiano E, Volsi SL, Chiara R, Sapienza M, Nigro L. Epidemiology and clinical features of cystic hydatidosis in Western Sicily: A ten-year review. World J Gastroenterol 2013; 19(48): 9351-9358
- URL: https://www.wjgnet.com/1007-9327/full/v19/i48/9351.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i48.9351
Human cystic echinococcosis (CE), or hydatid disease, is a parasitic zoonosis caused by the larval stages of the cestode Echinococcus granulosus. The definitive hosts of this parasite are usually members of the canid family, such as dogs, which develop the adult worm in the gut following ingestion of the larvae that are present in the tissues of the intermediate host. Following the ingestion of eggs that are expelled in the feces of the definitive host, larval cysts then go on to develop in the visceral tissue of the intermediate hosts (typically sheep and goats and occasionally, humans), particularly in the liver and lungs.
CE is found worldwide, especially where livestock breeding and farming are widespread and in areas where human, definitive and natural intermediate hosts are found in close proximity. In the human host, a hydatid cyst can lead to life-threatening complications, such as cyst rupture, with possible anaphylactic shock, the spread of new cysts, and bacterial infection. In Italy, Sicily is one of the most endemic areas for CE because of the high levels of farming and livestock breeding, with an average annual incidence of 3.2/100000 inhabitants[1]. CE is often under-diagnosed because it is frequently a silent condition that develops over several years and whose symptoms are only apparent when compression of internal organs occurs.
The aim of this study was to assess retrospectively the epidemiological and clinical characteristics of CE, and evaluate follow-up and response to treatment in patients affected by this disease.
From January 2000 to December 2010 all CE patients admitted to the Infectious Diseases Units of the University of Catania and of Basilotta Hospital in Nicosia-Enna were enrolled as participants in the study. Epidemiological, clinical and laboratory data were collected from the clinical records of each patient. Diagnosis of CE was made using clinical, imaging and laboratory parameters.
Epidemiological data included sex, age, race, job, place of residence, possible contact with stray dogs or hunting dogs, countryside activities, hunting and presence of other people in the family affected by CE. Patients were classified as being “exposed” or “not exposed” to CE on the basis of occupation and recreational activities potentially at risk of acquiring CE. Chest X-ray and abdominal ultrasound provided the parameters for an initial diagnosis of CE. None of the patients enrolled in this study were assessed using high-sensitivity tests, such as specific anti-Echinococcus immunoglobulin E (IgE)-ELISA Immuno-CAP or on the basis of micro/macroscopic morphology alone. Serum alanine aminotransferase (ALT), gamma-glutamyl transpeptidase (gamma-GT) and alkaline phosphatase (ALP) values were also collected.
Where appropriate, treatment consisted of albendazole at a dosage of 10-15 mg per kg per day, administered in two separate doses, each individual course of treatment lasting up to 3 mo. Ultrasound and X-ray assessed the response to treatment after 1 year. A complete “cure” was defined as the disappearance or the complete calcification of the cyst/s, thereby rendering them non-viable. “Improvement” was defined as a reduction in the diameter and/or number of cysts and/or partial calcification, and “impairment” as an increase in the diameter and/or number of cyst/s and the onset of relapses and/or complications. A “relapse” was defined as the onset of new cyst/s (regardless of location) and/or an increase in diameter of previously existing cyst/s.
Response to treatment was recorded together with details of any adverse events, the severity of such events and any further complications. Correlations between side effects and albendazole treatment were assessed using the Naranjo algorithm[2]. Side effect severity was measured using the FDA drug reaction severity scale[3].
As the average time before relapse was observed to be 30 ± 6.4 mo, for the purposes of calculating the relapse rate, the decision was made to include only those patients diagnosed before 2008. IgE titers were evaluated at diagnosis, at six months after beginning treatment and again in the case of relapse. Eosinophil percentages were evaluated at diagnosis and six mo after starting treatment. Hyper-eosinophilia was defined as an eosinophil percentage of ≥ 6%.
This study was conducted in accordance with the principles expressed in the Declaration of Helsinki. The Ethics Committee of the University of Catania approved the study. All patients provided written informed consent for their data to be analyzed.
Anti-echinococcus IgE was performed using ELISA Immuno-CAP (Phadia AB, Uppsala). Inferential statistical analysis was conducted using the χ2 test with Yates’ correction, Fishers’ exact test, Mann-Whitney’s U test and Student’s t-test.
Thirty-two patients were diagnosed with CE in the course of this study, of whom 21 were male and 11 were female, with a male-female ratio of 2:1. Their median age was 46 ± 16.5 years.
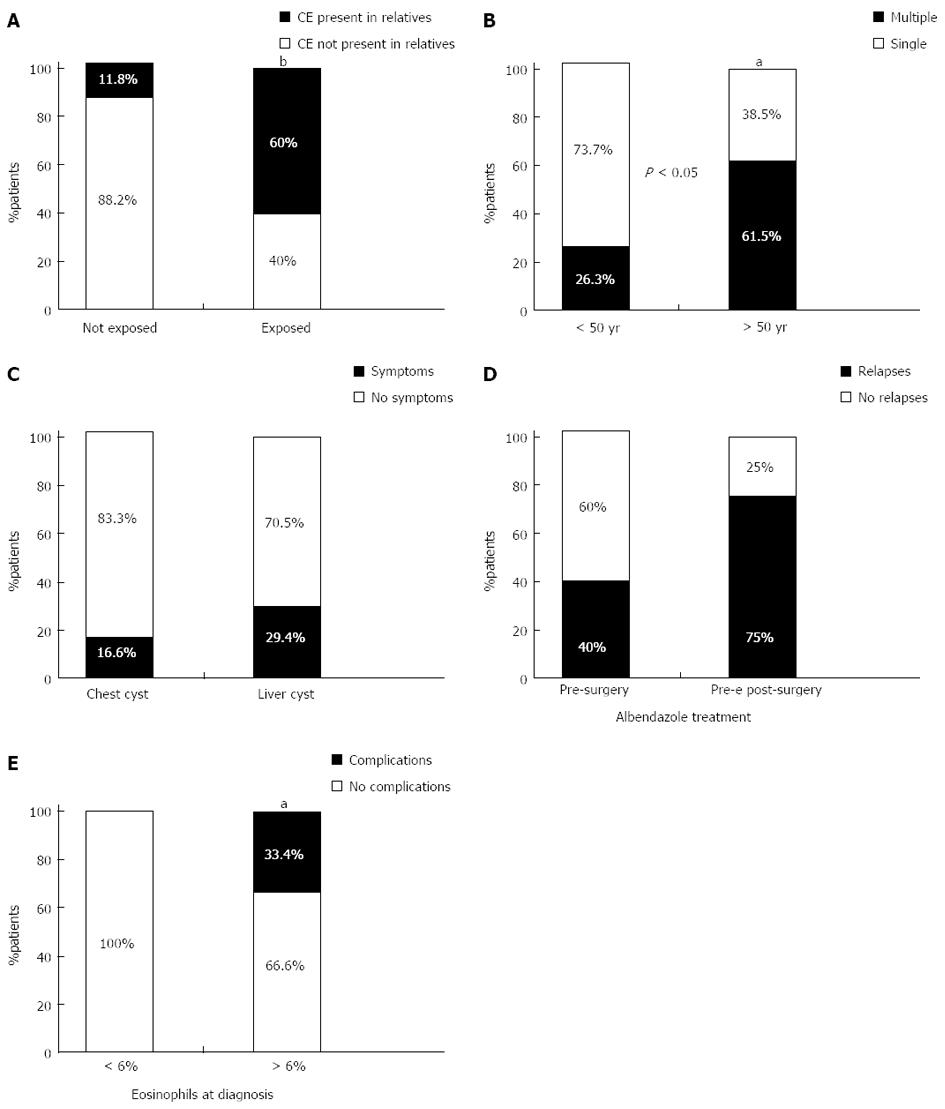
Fourteen patients, (43.7%) were classified as having been exposed to CE. In 12 of these cases (85.7%), exposure was a result of occupation (dog or cattle breeders, farmers, butchers, veterinarians), and in two cases (14.2%) exposure occurred during recreational activities (hunting). A strong correlation was observed between exposure to risk factors and the presence of other subjects affected by CE in the patients’ extended families (Figure 1A). Co-morbidities were observed in 17 (53.1%) patients, hypertension in nine, diabetes in four, chronic pulmonary diseases in two, hepatitis B in one and hepatitis C in one.
CE was detected in 16 patients (50%) by abdominal ultrasound, and in nine (28.1%) by chest X-ray, though in some cases diagnostic examinations were not carried out directly in the two units involved in the study. When diagnosed, 13 patients (40%) were observed as having a single hydatid cyst either in the liver (nine patients) or in the lung (four patients). Nineteen patients (60%) presented multiple cysts with multi-organ involvement. Multiple cysts were observed more frequently (73.7% vs 35.5%, P < 0.05) in patients below 50 years of age at the time of diagnosis (Figure 1B). When diagnosed, 24 patients (75%) were symptomatic, the most frequent symptom being a dry cough. The liver-lung cyst localization ratio was 1.8:1 and although pulmonary cysts tended to be more frequently symptomatic than hepatic cysts, this difference was not significant (Figure 1C).
Thirty patients were treated medically, receiving an average of two cycles of albendazole (6 mo treatment; interquartile range of two to four cycles). Sixteen patients were treated surgically, while 14 patients were treated both medically and surgically. Table 1 summarizes the main characteristics of the 32 patients affected by CE. Of the 30 medically treated patients, 10 developed severe adverse events: leukopenia was observed in four patients, pancytopenia in one patient, and an increase in ALT levels in five patients (for three of whom it resulted in the discontinuation of treatment). Gamma-GT and ALP levels were monitored during albendazole treatment and were found to be normal. The onset of adverse events was not related to ALT levels observed before treatment with albendazole. Of the 30 patients treated with albendazole alone or in combination with surgery, 23 had either a complete cure of CE (disappearance or calcification of cysts) or an improvement (reduction in either the number or size of cysts). An increase in cyst diameter (impairment) was observed in one patient. Four patients could not be evaluated for response to treatment because the cysts were surgically removed immediately after therapy. Two patients showed no improvement.
| Characteristics | Value |
| Age (yr) | |
| Median (range) | 46 ± 16.5 |
| Sex | |
| Male | 21 (65.7) |
| Female | 11 (34.3) |
| Risk factors | |
| Exposed | 14 (43.7) |
| Not exposed | 18 (56.3) |
| Comorbidities | |
| Affected | 17 (53.1) |
| Not affected | 15 (46.9) |
| Diagnosis | |
| Abdominal ultrasound | 16 (50) |
| Chest X-ray | 9 (28.1) |
| Unknown | 7 (21.9) |
| Hydatid cyst | |
| Single | 13 (40) |
| Multiple | 18 (60) |
| Treatment | |
| Medical | 30 (93.5) |
| Surgical | 16 (50) |
| Medical/surgical | 14 (46.8) |
Of the 21 patients diagnosed before 2005, five patients (23.8%) relapsed. Relapses tended to be more frequent in patients that were treated with albendazole before surgical treatment alone than in those treated both before and after surgery, although this difference was not statistically significant (Figure 1D).
Complications were observed in six patients (18.8%), these being cyst rupture, mostly traumatic, in five cases and bacterial infection in one case.
At diagnosis, 71% of patients were positive for specific CE IgE. The median value of anti-echinococcus IgE titer was 16 kUA/L (25th percentile = 2; 75th percentile = 24.3; min = 0; max = 34). Six months after beginning treatment with albendazole, 60% of patients were positive for specific IgE. A significant reduction in anti-echinococcus IgE titer values was also observed (IgE titer median = 1.2; 25th percentile = 0.75; 75th percentile = 9.5; min = 0; max = 369, P < 0.05). In cases of recurrence (five patients), the median value of anti-echinococcus IgE titer was 22 kUA/L (25th percentile = 4.93; 75th percentile = 38.5; min = 1.24; max = 42).
An eosinophil percentage of ≥ 6% was observed in 18 patients (56.3%) at diagnosis. After 6 mo of albendazole treatment, a significant reduction in eosinophil count was observed, with only three patients (12%) showing more than 6%. The median percentage value at diagnosis was 5% (25th percentile = 3; 75th percentile = 9; min = 0; max = 35.2), while 6 mo after beginning treatment with albendazole this has fallen to 2% (25th percentile = 1; 75th percentile = 3; min = 0.75; max = 12), with a highly significant difference in titres registered at these two times (P < 0.001).
Patients with hyper-eosinophilia at diagnosis developed complications, but not relapses, more frequently than other patients (33.4% vs 0%, P < 0.05) (Figure 1E). Patients with positive specific IgE results at diagnosis had a higher median eosinophil count than those with negative specific IgE.
As hydatid cysts usually develop slowly, CE is often an under-diagnosed (and consequently under-treated) disease. It is often difficult to assess its prevalence in a given area. The aim of this study was to assess the epidemiological and clinical characteristics of CE and to evaluate responses to therapy and follow-up by analyzing patterns in patients affected by the disease admitted to two Infectious Diseases Departments from 2000 to 2010.
During the ten-year observation period, 32 patients were diagnosed with CE, with a male/female ratio of 1.8/1. This ratio is comparable with that reported in other endemic areas and perhaps reflects the more frequent occupational exposure of males to the risk of infection[4-7]. The slow development of hydatid cysts, the nature of the host’s immune response, and the structure of the most typically affected tissues, may all contribute to late diagnosis[6,7].
Of the total number of patients, 43% were classified as having been exposed to CE through specific risk factors. This is probably caused by the sharing of occupational and/or recreational risk factors among patients and their relatives. It is, therefore, crucial to evaluate patient exposure in family contexts and suggest CE screening for relatives and co-workers.
Abdominal ultrasound was used as the primary diagnostic tool for 50% of patients. Ultrasound is widely considered one of the best hepatic diagnostic tools for CE, as it allows diagnosis even before the evidence of an increase in specific IgE antibodies[8,9]. In addition, abdominal ultrasound is fundamental for the staging of hepatic CE purposes and for establishing appropriate treatment options[10,11]. At the time of diagnosis, 60% of patients had multiple cysts. Those below the age of 50 were more likely to have multiple cysts (P < 0.05) than those diagnosed later. This probably reflected the fact that multiple cysts tend to become more symptomatic than single cysts.
In other studies, the majority of CE patients diagnosed during non-hospital based screening programs in endemic areas did not present any symptoms of CE[12]. By contrast, our patients presented CE-related symptoms in 75% of cases. This may reflect the higher probability that an already symptomatic patient is more likely to be referred to a hospital for investigation and/or treatment. In addition, a higher percentage of symptomatic patients was observed among patients affected by pulmonary-CE compared with hepatic-CE. This may be because hepatic hydatid cysts can grow for many years before symptom onset, while the symptoms of pulmonary CE tend to be more evident. Screening programs in endemic areas show a prevalence of hepatic-CE that is higher than pulmonary-CE, with a liver:chest localization ratio of 6-12:1[12]. In the patients of this study, the ratio was around 2.5:1, thus confirming the greater probability of finding cases of pulmonary-CE rather than hepatic CE in a hospital setting[12].
The patients in this study were given albendazole in 93.8% of cases, with an average treatment duration of 6 mo and a maximum of 120 mo in one patient affected by non-surgical multi-organ CE. Studies have shown that Albendazole is more effective than mebendazole in the treatment of CE[13]. Some studies suggest treating CE patients with albendazole for no less than 3 mo. but no longer than 6 mo, longer treatment being offered only to patients with non-abdominal and non-pulmonary CE[14,15].
Response to medical treatment was evaluated by means of ultrasound and X-ray, performed 1 year after beginning of therapy with albendazole. A complete cure (disappearance or calcification of cysts) or improvement (reduction in number or size of cysts) was achieved in 76% of patients treated with albendazole alone or in combination with surgery. This confirmed the results from other studies conducted on around 2000 CE patients treated with benzimidazoles, which showed a response to therapy of 50%-70% after a 12-mo follow-up[16-18]. Albendazole-related adverse events are usually self-limiting and rarely severe. However, in CE, factors such as the longer period of treatment needed, the frequently older age of patients and drug-cyst interaction, may lead to higher occurrence and severity[17-20]. One third of the patients in the current study developed moderate to severe adverse events. In three of the five hypertransaminasemia cases, this led to the discontinuation of treatment with this drug. Similarly, in a study carried out by Gil-Grande et al[15], 10%-20% of CE patients were seen to develop hypertransaminasemia, which was always reversible after drug discontinuation or during the pauses between albendazole cycles[13-19].
The surgical treatment of CE can potentially lead to a complete cure of the disease and is indicated in the following cases: for the removal of large cysts with multiple daughter vesicles; for cysts exerting pressure on adjacent vital organs; in the case of cysts communicating with the biliary tree (as an alternative to percutaneous treatments or PTs); or where there are single superficial liver cysts and infected cysts (when PTs are not possible). In non-surgical cases, alternative techniques such as Puncture Aspiration Injection Respiration (PAIR) can be considered[6]. In our study, 50% of the patients were surgically treated, but only half of these cases were given albendazole both pre- and post-surgery. The WHO recommendations stipulate that albendazole should be given at least 4 d before surgery and for 3 mo following surgery[20]. Other studies suggest that a 3 mo albendazole cycle before surgery is more effective in reducing cyst vitality, rather than treatment for only 4 d or even one mo[15]. Appropriate post-surgical albendazole therapy has also been observed to prevent CE relapses because of surgical dissemination[13-24].
Among the patients of this study, 60% of those treated with albendazole before surgery alone suffered a relapse, compared to only 25% of those treated both before and after surgery. This suggests that appropriate medical therapy offered to the patient both pre- and post-surgery is necessary.
The literature reports a relapse incidence in the treatment of CE of between 0% and 30%, with an average time for the onset of relapse symptoms of 3-4 years[21]. The patients in this study developed symptomatic relapses, hepatic in 80% of cases and pulmonary in 20% of cases, in an average time of 20.7 mo after the cessation of treatment or following surgery, with a relapse rate of 23.8%.
Six of the patients in this study (18.8%) developed complications, these being bacterial super-infection of a cyst in one case and cyst rupture (mostly traumatic and easily managed using a surgical approach) in five cases. The most frequent complication reported in CE endemic areas is cyst rupture into the biliary tree (5%-17%), followed by super-infection (5.1%)[25,26].
ELISA for specific anti-echinococcus IgG and IgE titer detection is frequently used in CE serological diagnosis and screening, as it requires a short preparation time, has a relatively limited cost, and shows a sensitivity and specificity of 95%[27-30]. In our study, at the time of diagnosis, 71% of patients were positive for specific CE IgE, with a median titer of 16 kUA/ which dropped significantly to 1.2 kUA/ (with 60% of positive specific IgE patients) 6 mo after beginning treatment with albendazole. This confirmed that monitoring IgE titer variation over time is likely to be more helpful in evaluating CE activity than considering single absolute values[31]. However, it should be noted that fluctuations in specific IgE titer that are unrelated to the clinical stage of the disease are possible. Extremely prolonged positivity (e.g., 3-7 years) is possible even after surgical cyst treatment[32-34]. Persistence of high specific IgE titer beyond 3-7 years usually indicates the onset of a relapse. This appeared to be confirmed by the patients of this study that developed relapses: their median specific IgE value was 22 kUA/ at the time of relapse diagnosis[32-34]. On this basis, it is possible to claim that in CE-affected patients, specific IgE titer detection can be considered as a useful tool in post-therapeutic follow-up to predict cure and eventual relapse.
In the literature, CE diagnosis and follow-up eosinophil cell counts have usually been considered of limited value because it is significantly high in no more than half of CE affected patients, as confirmed in our own study[4]. In this study, although 56.3% of patients presented a percentage value of eosinophils ≥ 6% (defined as hyper-eosinophilia) at diagnosis, six months after beginning treatment with albendazole, only 12% of patients continued to display hyper-eosinophilia. At diagnosis, the median percentage value of eosinophils was 5%; however, 6 mo after beginning treatment with albendazole, this value had dropped to 2%. This significant difference in titers registered at the two different times (P < 0.001) may be a result of the positive response to therapy obtained in the majority of patients. Finally, we observed that patients with positive specific IgE at diagnosis had a higher median eosinophil percentage than those with negative specific IgE (P < 0.05). This suggested that, in settings with limited resources, or where specific IgE detection is not feasible, eosinophil percentage value at diagnosis could be used as an effective tool for post-therapeutic follow-up, to assess the response to therapy and as an aid in patient prognosis.
In conclusion, on the basis of our data, three main proposals can be made. Firstly, we advocate the routine screening for CE in relatives (and/or close associates) of patients classified as being exposed to CE in settings where this is feasible. We also recommend the use of anti-echinococcus IgE titer as a response marker of albendazole therapy (in low resource settings this could be replaced by eosinophil percentage value). Our results also highlight the need for an extended and well managed period of follow-up after surgical and/or medical therapy, as indicated by the average time for the onset of relapse, which in our patients was about 2 years. Finally, while the surgical approach remains the first-line treatment in most CE cases, appropriate medical therapy before and after surgery should also be considered a useful tool to reduce the incidence of relapse[35].
Endemic in sheep raising areas, cystic echinococcosis (CE) is a neglected disease. This neglect is also reflected in the clinical management of CE, which has evolved over the last few decades with little or no comparative evaluation of the efficacy, effectiveness, rate of adverse events and relapse rates. In addition, CE is often under-diagnosed as it is frequently undetected, developing over the course of several years.
On the basis of their data, some proposal can be made: (1) routine screening for CE in relatives (and/or close associates) of patients exposed, according to occupation and recreational activities, who are potentially at risk for acquiring CE, to speed up CE diagnosis; (2) extended and manage the period of follow-up after surgical and/or medical therapy to avoid late CE relapse diagnosis; and (3) administer appropriate medical therapy before and after surgery to reduce the incidence of relapse.
These findings suggest that anti-echinococcus immunoglobulin E (IgE) titer may be successfully replaced by eosinophil percentage count in low resource settings as a marker of response to therapy.
Cystic echinococcosis is a helminthic zoonosis that can affect humans, most frequently involving the liver or the lungs. Symptoms typically show themselves only when the compression of internal organs occurs. In humans, the natural history of CE can lead to life-threatening complications, such as cyst rupture (with possible anaphylactic shock), the spread of new cysts, and bacterial infection.
Cystic echinococcosis is a helminthic zoonosis that can affect human beings, most frequently involving the liver or the lungs, showing symptoms only when the compression of internal organs occurs. In humans, the natural history of CE can lead to life-threatening complications, such as cyst rupture, with possible anaphylactic shock, the spread of new cysts, and bacterial infection.
Cystic echinococcosis is a helminthic zoonosis that can affect humans, most frequently involving the liver or the lungs. Symptoms typically show themselves only when the compression of internal organs occurs. In humans, the natural history of CE can lead to life-threatening complications, such as cyst rupture (with possible anaphylactic shock), the spread of new cysts, and bacterial infection.
P- Reviewers: Amiri M, Mendes RE S- Editor: Gou SX L- Editor: Stewart GJ E- Editor: Liu XM
| 1. | De Carneri. Parassitologia generale e umana. 13th ed. Casa Editrice Ambrosiana. Rozzano (MI): Cea 2013; 265-394. [Cited in This Article: ] |
| 2. | Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7061] [Cited by in F6Publishing: 7670] [Article Influence: 178.4] [Reference Citation Analysis (0)] |
| 3. | Available from: http://www.fda.gov/Safety/MedWatch/default.htm. [Cited in This Article: ] |
| 4. | El Marsfy YS, Morsy TA. A preliminary study on echinococcosis in Riyadh, Saudia Arabia. J Pak Med Assoc. 1975;25:10-11. [PubMed] [Cited in This Article: ] |
| 5. | Amr SS, Amr ZS, Jitawi S, Annab H. Hydatidosis in Jordan: an epidemiological study of 306 cases. Ann Trop Med Parasitol. 1994;88:623-627. [PubMed] [Cited in This Article: ] |
| 6. | Fahim F, Al Salamah SM. Cystic echinococcosis in Central Saudi Arabia: a 5-year experience. Turk J Gastroenterol. 2007;18:22-27. [PubMed] [Cited in This Article: ] |
| 7. | Culafic DJ, Katic-Radivojevic S, Kerkez M, Vukcevic M, Rankovic V, Stefanovic D. Liver cystic echinococcosis in humans - a study of 30 cases. Helminthologia. 2007;44:157-161. [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Shambesh MA, Craig PS, Macpherson CN, Rogan MT, Gusbi AM, Echtuish EF. An extensive ultrasound and serologic study to investigate the prevalence of human cystic echinococcosis in northern Libya. Am J Trop Med Hyg. 1999;60:462-468. [PubMed] [Cited in This Article: ] |
| 9. | Ozkol M, Kilimcioğlu AA, Girginkardeşler N, Balcioğlu IC, Sakru N, Korkmaz M, Ok UZ. A discrepancy between cystic echinococcosis confirmed by ultrasound and seropositivity in Turkish children. Acta Trop. 2005;93:213-216. [PubMed] [Cited in This Article: ] |
| 10. | WHO Informal Working Group. International classification of ultrasound images in cystic echinococcosis for application in clinical and field epidemiological settings. Acta Trop. 2003;85:253-261. [PubMed] [Cited in This Article: ] |
| 11. | WHO Informal Working Group. PAIR: Puncture, Aspiration, Injection, Re-Aspiration. An option for the treatment of Cystic Echinococcosis. WHO/CDS/CSR/APH/2001.6. Available from: http://whqlibdoc.who.int/hq/2001/WHO_CDS_CSR_APH_2001.6.pdf. [Cited in This Article: ] |
| 12. | Larrieu EJ, Frider B. Human cystic echinococcosis: contributions to the natural history of the disease. Ann Trop Med Parasitol. 2001;95:679-687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Franchi C, Di Vico B, Teggi A. Long-term evaluation of patients with hydatidosis treated with benzimidazole carbamates. Clin Infect Dis. 1999;29:304-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 146] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Horton RJ. Chemotherapy of Echinococcus infection in man with albendazole. Trans R Soc Trop Med Hyg. 1989;83:97-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 153] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Gil-Grande LA, Rodriguez-Caabeiro F, Prieto JG, Sánchez-Ruano JJ, Brasa C, Aguilar L, García-Hoz F, Casado N, Bárcena R, Alvarez AI. Randomised controlled trial of efficacy of albendazole in intra-abdominal hydatid disease. Lancet. 1993;342:1269-1272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 173] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | WHO/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. In: Eckert J, Gemmell MA, Meslin FX, Pawlowski ZS, editors. World Organization for Animal Health, Paris, France, 2001. Available from: http://whqlibdoc.who.int/publications/2001/929044522X.pdf. [Cited in This Article: ] |
| 17. | Horton RJ. Albendazole in treatment of human cystic echinococcosis: 12 years of experience. Acta Trop. 1997;64:79-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 187] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Horton J. Albendazole for the treatment of echinococcosis. Fundam Clin Pharmacol. 2003;17:205-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Brunetti E, Kern P, Vuitton DA. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010;114:1-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1108] [Cited by in F6Publishing: 1152] [Article Influence: 82.3] [Reference Citation Analysis (0)] |
| 20. | WHO/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. World Organization for Animal Health, Paris, France, 2001. . [Cited in This Article: ] |
| 21. | Guidelines for treatment of cystic and alveolar echinococcosis in humans. WHO Informal Working Group on Echinococcosis. Bull World Health Organ. 1996;74:231-242. [PubMed] [Cited in This Article: ] |
| 22. | Topçu S, Kurul IC, Taştepe I, Bozkurt D, Gülhan E, Cetin G. Surgical treatment of pulmonary hydatid cysts in children. J Thorac Cardiovasc Surg. 2000;120:1097-1101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Cetin G, Doğan R, Yüksel M, Alp M, Uçanok K, Kaya S, Unlü M. Surgical treatment of bilateral hydatid disease of the lung via median sternotomy: experience in 60 consecutive patients. Thorac Cardiovasc Surg. 1988;36:114-117. [PubMed] [Cited in This Article: ] |
| 24. | Isitmangil T, Sebit S, Tunc H, Gorur R, Erdik O, Kunter E, Toker A, Balkanli K, Ozturk OY. Clinical experience of surgical therapy in 207 patients with thoracic hydatidosis over a 12-year-period. Swiss Med Wkly. 2002;132:548-552. [PubMed] [Cited in This Article: ] |
| 25. | Kune G, Morris DI. Hydatid disease. In: Schwartz SI, Ellis H, Husser WC, editors. Maingot’s Abdominal Operations. 9th ed. Appleton & Lange 1989; 1225-1240. [Cited in This Article: ] |
| 26. | Kattan YB. Intrabiliary rupture of hydatid cyst of the liver. Ann R Coll Surg Engl. 1977;59:108-114. [PubMed] [Cited in This Article: ] |
| 27. | Barros JL. Hydatid disease of the liver. Am J Surg. 1978;135:597-600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 90] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | el-On J, Khaleel E, Malsha Y, Nahmias J, Schantz P, Sneir R, Ben-Ismail R, Furth M, Hoida G. Echinococcus granulosus: a seroepidemiological survey in northern Israel using an enzyme-linked immunosorbent assay. Trans R Soc Trop Med Hyg. 1997;91:529-532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Ambroise-Thomas P, Desgeorges PT. [Diagnositc value and limitations of the ELISA test applied to hydatidosis]. Bull Soc Pathol Exot Filiales. 1980;73:89-99. [PubMed] [Cited in This Article: ] |
| 30. | Iacona A, Pini C, Vicari G. Enzyme-linked immunosorbent assay (ELISA) in the serodiagnosis of hydatid disease. Am J Trop Med Hyg. 1980;29:95-102. [PubMed] [Cited in This Article: ] |
| 31. | Biffin AH, Jones MA, Palmer SR. Human hydatid disease: evaluation of an ELISA for diagnosis, population screening and monitoring of control programmes. J Med Microbiol. 1993;39:48-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Riganò R, Profumo E, Siracusano A. New perspectives in the immunology of Echinococcus granulosus infection. Parassitologia. 1997;39:275-277. [PubMed] [Cited in This Article: ] |
| 33. | Rickard MD. Serological diagnosis and post-operative surveillance of human hydatid disease. I. Latex agglutination and immunoelectrophoresis using crude cyst fluid antigen. Pathology. 1984;16:207-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Ravinder PT, Parija SC, Rao KS. Evaluation of human hydatid disease before and after surgery and chemotherapy by demonstration of hydatid antigens and antibodies in serum. J Med Microbiol. 1997;46:859-864. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Ayles HM, Corbett EL, Taylor I, Cowie AG, Bligh J, Walmsley K, Bryceson AD. A combined medical and surgical approach to hydatid disease: 12 years’ experience at the Hospital for Tropical Diseases, London. Ann R Coll Surg Engl. 2002;84:100-105. [PubMed] [Cited in This Article: ] |