Published online Dec 14, 2013. doi: 10.3748/wjg.v19.i46.8722
Revised: October 5, 2013
Accepted: November 2, 2013
Published online: December 14, 2013
Processing time: 150 Days and 10.2 Hours
AIM: To investigate the effect of somatostatin and dexamethasone on early postoperative small bowel obstruction with obliterative peritonitis (EPSBO-OP).
METHODS: This prospective randomized study included 70 patients diagnosed with EPSBO-OP from June 2002 to January 2009. Patients were randomized into two groups: a control group received total parenteral nutrition and nasogastric (NG) tube feeding; and an intervention group received, in addition, somatostatin and dexamethasone treatment. The primary endpoints were time to resolution of bowel obstruction and length of hospital stay, and the secondary endpoints were daily NG output and NG feeding duration, treatment-related complications, postoperative obstruction relapse, and patient satisfaction.
RESULTS: Thirty-six patients were allocated to the intervention group and 34 to the control group. No patient needed to undergo surgery. Patients in the intervention group had an earlier resolution of bowel obstruction (22.4 ± 9.1 vs 29.9 ± 10.1 d, P = 0.002). Lower daily NG output (583 ± 208 vs 922 ± 399 mL/d, P < 0.001), shorter duration of NG tube use (16.7 ± 8.8 vs 27.7 ± 9.9 d, P < 0.001), and shorter length of hospital stay (25.8 vs 34.9 d, P = 0.001) were observed in the intervention group. The rate of treatment-related complications (P = 0.770) and relapse of obstruction (P = 0.357) were comparable between the two groups. There were no significant differences in postoperative satisfaction at 1, 2 and 3 years between the two groups.
CONCLUSION: Somatostatin and dexamethasone for EPSBO-OP promote resolution of obstruction and shorten hospital stay, and are safe for symptom control without increasing obstruction relapse.
Core tip: This prospective study revealed that somatostatin and dexamethasone, when used in combination, promoted the resolution of small bowel obstruction and shortened length of hospital stay in patients with early postoperative small bowel obstruction due to obliterative peritonitis. Somatostatin and dexamethasone were effective in symptom control in this population.
- Citation: Gong JF, Zhu WM, Yu WK, Li N, Li JS. Conservative treatment of early postoperative small bowel obstruction with obliterative peritonitis. World J Gastroenterol 2013; 19(46): 8722-8730
- URL: https://www.wjgnet.com/1007-9327/full/v19/i46/8722.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i46.8722
Early postoperative small bowel obstruction (EPSBO) with obliterative peritonitis (EPSBO-OP), or “frozen abdomen”, (also known as early postoperative inflammatory small bowel obstruction[1,2], is caused by dense, vascular and inseparable inflammatory adhesions in response to multiple sequential laparotomies, surgery for enterocutaneous fistula (ECF), or extensive adhesiolysis[3-6]. Patients with EPSBO-OP may often have a combination of partial mechanical obstruction and diffuse small bowel and colonic ileus. Surgery attempting to lyse the adhesions in these patients is unsuitable due to the high risk of iatrogenic injuries such as ECF or massive small bowel resection[7].
The traditional approach to managing these patients is total parenteral nutrition (TPN) and observation, and most obstructions are relieved spontaneously[8]. However, it often takes a long period (i.e., several weeks to months) before the bowel function recovers[9], and it is associated with high costs and high risk of PN-related complications. Patients have to tolerate prolonged nasogastric (NG) suction and fluid loss, which can also create discomfort and complications.
Somatostatin is well known for its antisecretory function in the intestinal epithelium, and clinical studies have suggested that it may be useful for symptomatic relief and treatment of bowel obstruction[10,11]. Dexamethasone is a frequently used synthetic corticosteroid that reduces intraperitoneal adhesion and inflammatory edema[12,13], and is effective in promoting the resolution of malignant bowel obstruction or obstruction with encapsulating peritoneal sclerosis[14,15]. Based on their mechanisms of action and results of previous studies, we hypothesized that these two drugs, when used in combination, would be beneficial in reducing gastrointestinal secretion and promoting the regression of inflammation and adhesion in patients with EPSBO-OP. However, comparative studies of the effect of somatostatin and dexamethasone in EPSBO-OP are lacking.
In the current study, we prospectively analyzed a consecutive series of patients with ESPBO-OP in our department, a tertiary gastrointestinal referral center in China. The aim of the study was to evaluate the effect of somatostatin and dexamethasone on length of hospital stay and symptom control in patients with ESPBO-OP.
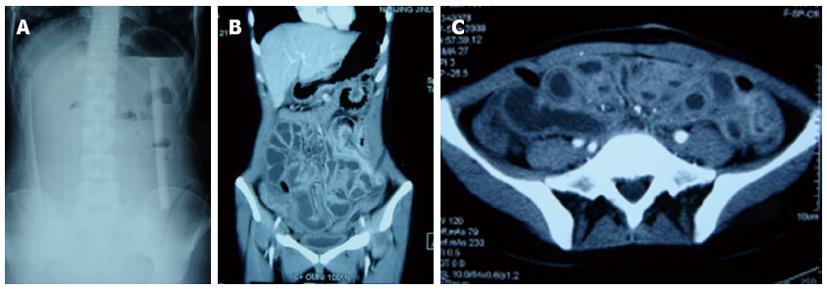
The diagnostic criteria for ESPBO-OP were: (1) intestinal obstruction that developed 1-4 wk postoperatively after initial recovery of postoperative ileus, as defined previously[16,17]; (2) typical operative history with extensive enterolysis or repeated laparotomy over a short period; (3) absence of severe colicky abdominal pain, but with obstipation, abdominal distension, nausea and vomiting; (4) palpation of a subincisional or whole abdominal mass on physical examination, with only mild or no tenderness on palpation, no peritoneal signs, and low-pitched or no bowel tones; and (5) low or absent air-fluid levels on upright abdominal film, edematous and thickened bowel wall with unclear borders on abdominal computed tomography (CT), and fluid-filled lumen with paucity of gas.
Exclusion criteria included: patients aged < 18 years; patients with terminal disease or presence of metastatic cancer; CT or X-ray film suggesting local adhesions, intussusceptions, volvulus, internal hernia, intra-abdominal abscesses, or hematoma; patients with suspicion of mechanical bowel obstruction, paralytic ileus, or idiopathic pseudo-obstruction; and patients with hypokalemia and retroperitoneal injury that may cause paralytic ileus. On previous laparotomy, all adhesions should have been freed and the possibility of mechanical obstruction, such as anastomotic stenosis or residual malignancy, excluded. All radiographs (X-ray and CT scan) were extrapolated by a specialist in gastrointestinal radiology.
Assignments were based on computer-generated randomizations that were kept in sealed, sequentially numbered envelopes until used. After a diagnosis of EPSBO-OP was made, patients were randomly assigned into one of the two groups: TPN group (T group) or TPN + dexamethasone + somatostatin group (TDS group). The study was approved by the ethics committee of the hospital, and all patients provided written informed consent before enrollment.
Nil by mouth and nasogastric tube were introduced for all patients. For patients in the control group (T group), a central venous catheter was placed on admission. After fluid resuscitation and correction of electrolyte abnormalities, patients were infused with TPN from all-in-one bags. The amount of non-protein calories (NPCs) was 20-25 kcal/kg per day or determined by indirect calorimetry. The NPCs consisted of 60%-70% carbohydrate, with the ratio of NPC: nitrogen = 120-140:1. Parenteral antibiotics were administered when leukocytosis was present. The amount of intravenous fluid was adjusted to maintain optimal hydration and sufficient urine output (> 1 L/d).
The duration of NG tube feeding depended on daily output. If daily NG output was < 200 mL for 2 d, the NG tube was clamped. The NG tube was removed if the patient was able to tolerate for 12 h after clamping. After patients resumed oral intake, gastrointestinal prokinetics (mosapride, 5 mg/8 h, Gasmotin; Dainippon Sumitomo Pharma Co. Ltd., Osaka, Japan) was given until discharge.
In the intervention group (TDS group), in addition to the treatment protocol in the control group, somatostatin (Stilamin; Merck-Serono S.A., Geneva, Switzerland) was given at 6 mg/d by continuous intravenous infusion. The criteria for stopping NG tube usage were similar to those for the T group, while somatostatin was stopped within 24 h after the patient defecated or passed gas. Dexamethasone sodium phosphate (5 mg/mL, Lukang Pharmaceuticals, Shandong, China) was used since the first day of treatment with an intravenous dosage of 5 mg/8 h for seven consecutive days, then 2.5 mg/12 h for 1 d, and stopped. If the patient defecated or passed gas in < 8 d after treatment, dexamethasone was withdrawn within 24 h after resolution of obstruction. During treatment, patients were carefully monitored for abdominal symptoms and systemic complications, such as cholestasis, central catheter infections, and systemic infections. Other complications, such as hypovolemia, electrolyte-fluid imbalance, and hyperglycemia, were corrected during treatment and not documented.
Indications for prompt surgery included suspicion for strangulation (continuous vs colicky pain, fever, tachycardia, peritoneal signs, and sustained leukocytosis), or clinical deterioration that implied failure of conservative management for > 3 mo.
The following parameters were recorded in each patient: age and sex; interval between symptom onset and the most recent laparotomy; clinical features including symptoms, presence or absence of fever, white blood cells, nutritional status, and comorbidity; procedures and duration of last operation; and time of previous laparotomy. Complete resolution of obstruction was established when symptoms and signs of obstruction subsided, normal flatus and defecation returned, and there was no relapse of obstructive symptoms after withdrawal of somatostatin. Then, liquid food or enteral nutrition was started. A semiliquid food was usually given 2 d later. Patients were discharged when intravenous fluid was stopped and semiliquid food was tolerated for 3 d.
The primary endpoints of the study were time to resolution of obstruction and length of hospital stay, and the secondary endpoints were daily NG output, NG tube placement duration, treatment-related complications, postoperative obstruction relapse, and patient satisfaction.
Sample size calculation was based on our previous data of historical comparison[18], which showed a mean 26.0 d for the intervention group and 30.3 d for the control group, with mean ± SD of 9.0 d. Approximately 35 patients in each group were needed to detect a difference in hospital stay with 80% power and a two-sided 5% significance level.
The patients were followed for ≥ 3 years after discharge. At each 6-mo visit, patients were given a questionnaire that was completed and returned by mail or they were contacted by telephone with the complete questions answered. Obstruction relapse was defined as abdominal pain with the halt of flatus, and nausea/vomiting, which needed further medical treatment and admission to hospital. At 1, 2 and 3 years, the degree of postoperative satisfaction was evaluated in every patient by using a unified scale (1-4) that indicated very unsatisfied, unsatisfied, satisfied, and very satisfied, respectively. Patient satisfaction was based on the core symptoms of the Gastrointestinal Quality of Life Index such as abdominal pain, feeling of abdominal distension, flatus and stool frequency, anorexia, fatigue, and nausea and vomiting[19]. The definition of “very satisfied” was the presence of none of the above-mentioned gastrointestinal symptoms in the past year; “satisfied” was occasional gastrointestinal symptoms; “unsatisfied” was several episodes of abdominal symptoms in the past year, and “very unsatisfied” was frequent abdominal symptoms.
Statistical analysis was performed by per-protocol analysis. Quantitative variables, presented as mean ± SD (range), were analyzed by Mann-Whitney U test or Student’s t test if appropriate. Quantitative variables, expressed as a number (percentage), were analyzed by Pearson’s χ2 test or Fisher’s exact test. All analyses were performed with SPSS version 13.0 (SPSS, Chicago, IL, United States). P < 0.05 indicated statistical significance.
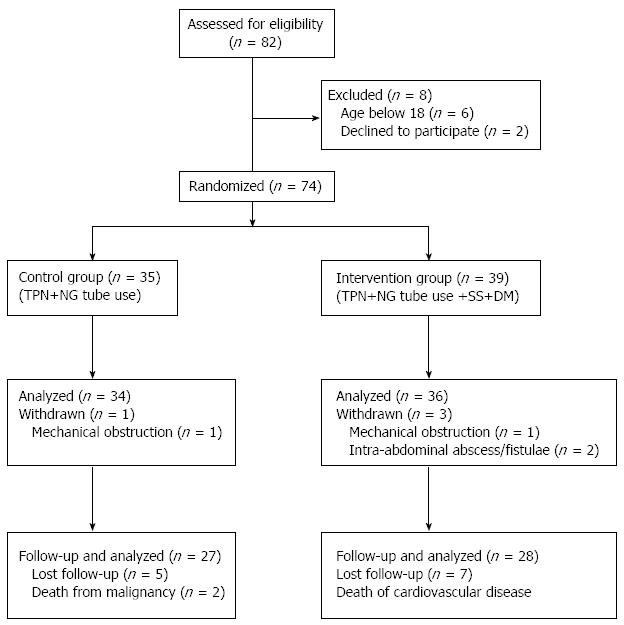
Between June 2002 and January 2009, 82 patients were diagnosed with EPSBO-OP in our department. Six patients were aged < 18 years and two declined to participate in the study, which left 74 patients enrolled in the study. Two patients were eventually confirmed to have mechanical obstruction and two had intra-abdominal abscesses or anastomotic fistulae and withdrew from the study. The dropout patients were eventually proven not to have EPSBO-OP, therefore, we used per-protocol analysis instead of intention-to-treat statistical analysis. Therefore, 70 cases were evaluated (34 in the T group and 36 in the TDS group) (Figure 1).
Patients’ demographic data and previous surgeries are listed in Tables 1 and 2. Fifteen patients (10 in the TDS group and 5 in the T group) had a history of malignancy but all underwent radical surgical resection. There were no significant differences between the two groups with respect to laboratory and clinical features at trial entry. The median onset of obstructive symptoms was postoperative day 9.4 ± 3.5 (range: 5-23 d).
| T group (n = 34) | TDS group (n = 36) | P value | |
| Age (yr) | 43.9 ± 10.2 (26-64) | 45.4 ± 13.2 (20-78) | 0.597 |
| Sex ratio (M/F) | 24/10 | 18/18 | 0.079 |
| Symptoms onset | |||
| ≤ 1 POW | 12 (35.3) | 13 (36.1) | 0.943 |
| 1-2 POW | 19 (55.9) | 21 (58.3) | 0.836 |
| 2-3 POW | 3 (8.8) | 1 (2.8) | 0.276 |
| 3-4 POW | 0 | 1 (2.8) | |
| Mean POD of symptom onset | 9.8 ± 3.4 (5-19) | 9.1 ± 3.5 (5-23) | 0.412 |
| Symptoms | |||
| Nausea and vomiting | 32 (94.1) | 33 (91.7) | 0.691 |
| Abdominal distension | 23 (67.6) | 20 (55.6) | 0.299 |
| Colic pain | 0 | 0 | |
| Obstipation | 34 (100) | 36 (100) | |
| Hyperthermia (> 37.5 °C)1 | 1 (2.9) | 1 (2.6) | 0.967 |
| Maximum WBC (× 109/L) | 7.0 ± 2.3 (3.7-12.3) | 8.0 ± 3.0 (4.8-17.1) | 0.148 |
| Neutrophil (%) | 71.2 ± 10.4 (48-88) | 75.1 ± 8.4 (59-91) | 0.084 |
| Nutrition status on admission | |||
| Mean BMI (kg/m2) | 20.4 ± 2.2 (16.8-26.4) | 21.2 ± 2.8 (17.3-28.0) | 0.248 |
| Hypoalbuminemia (< 35g/L) | 13 (38.2) | 8 (22.2) | 0.144 |
| Anemia (Hb < 120g/L) | 10 (29.4) | 4 (11.1) | 0.056 |
| Comorbidity | 3 (8.8) | 4 (11.1) | 0.750 |
| T group (n = 34) | TDS group (n = 36) | P value | |
| No. of laparotomies | |||
| 1 | 3 (8.8) | 4 (11.1) | 0.750 |
| 2 | 7 (20.6) | 11 (30.6) | 0.340 |
| 3 | 15 (44.1) | 11 (30.6) | 0.241 |
| 4 | 6 (17.6) | 4 (11.1) | 0.435 |
| ≥ 5 | 3 (8.8) | 6 (16.7) | 0.327 |
| Mean No. (range) | 3.0 ± 1.0 (1-5) | 2.9 ± 1.3 (1-6) | 0.927 |
| Type of last operation | |||
| Bowel obstruction | 12 (35.3) | 18 (50.0) | 0.214 |
| Enterocutaneous fistula | 15 (44.1) | 10 (27.8) | 0.154 |
| Enterectomy/colectomy | 2 (5.9) | 1 (2.8) | 0.522 |
| Gastroduodenal surgery | 0 | 1 (2.8) | 0.328 |
| Hematoma removal | 2 (5.9) | 1 (2.8) | 0.522 |
| Appendectomy1 | 0 | 2 (5.6) | 0.163 |
| Laparotomy after trauma | 1 (2.9) | 1 (2.8) | 0.967 |
| Others | 2 (5.9) | 2 (5.6) | 0.953 |
| Patients with history of malignancy | 5 (14.7) | 10 (27.8) | 0.183 |
| At last operation | |||
| with extensive enterolysis2 | 24 (70.6) | 30 (83.4) | 0.204 |
| with diffuse peritonitis | 4 (11.8) | 2 (5.6) | 0.354 |
| < 2 wk from previous surgery | 3 (8.8) | 3 (8.3) | 0.971 |
| Last operation time (h) | 4.8 ± 1.1 (2.0-6.5) | 4.1 ± 1.3 (1.5-7) | 0.041 |
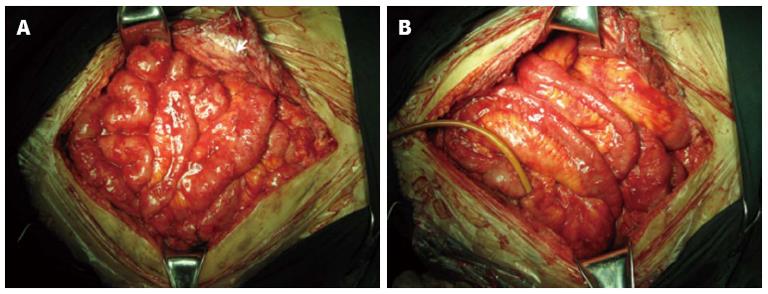
Sixty-three patients underwent more than two operations before EPSBO-OP developed. At last surgery, extensive adhesiolysis (including intestinal splinting[20]) was performed in 54 (77.1%) of the operations; six (8.6%) received repeated laparotomy within 2 wk, and another six patients had diffuse peritonitis during last laparotomy. Although the mean number of operations (2.9 ± 1.3 vs 3.0 ± 1.0, P = 0.927) and type of operation were similar between the two groups, the operation time was shorter in the TDS group compared with the T group (4.1 ± 1.3 vs 4.8 ± 1.1 h, P = 0.041). Typical radiographic and intraoperative findings at the last operation are shown in Figures 2 and 3, respectively.
Treatment was successful for all patients in both groups. The mean length of hospital stay was 30.5 ± 10.9 (16-69) d. No patients withdrew because they needed surgery for strangulation or failure of conservative therapy. There were no deaths during treatment. In the TDS group, the mean duration of somatostatin usage was 23.5 ± 9.1 (14-53) d, while dexamethasone was used for 8 d in all patients.
As shown in Table 3, somatostatin and dexamethasone had a marked effect on the recovery of bowel function, as indicated by earlier passage of stool or gas (22.4 ± 9.1 vs 29.9 ± 10.1 d, P = 0.002). The length of hospital stay in the intervention group was shorter than in the control group (25.8 ± 9.9 vs 34.7 ± 11.2 d, P = 0.001).
| TPN (n = 34) | TDS (n = 36) | P value | |
| Time to obstruction resolution (d) | 29.9 ± 10.1 (17-60) | 22.4 ± 9.1 (13-52) | 0.002 |
| Length of hospital stay (d) | 34.7 ± 11.2 (21-69) | 25.8 ± 9.9 (16-57) | 0.001 |
| Daily NG output (mL)1 | 922 ± 399 (400-1825) | 583 ± 208 (150-1050) | < 0.001 |
| Mean NG duration (d) | 27.7 ± 9.9 (7-54) | 16.7 ± 8.8 (3-42) | < 0.001 |
| Relapse of obstruction | |||
| 1 yr after operation (n/N)2 | 3/32 | 2/34 | 0.668 |
| 2 yr after operation (n/N)3 | 6/29 | 5/31 | 0.745 |
| 3 yr after operation (n/N)4 | 8/27 | 6/28 | 0.547 |
| Postoperative satisfaction ≥ 35 | |||
| 1 yr after operation (n/N)2 | 22/32 | 20/34 | 0.451 |
| 2 yr after operation (n/N)3 | 15/29 | 14/31 | 0.796 |
| 3 yr after operation (n/N)4 | 11/27 | 10/28 | 0.785 |
The daily NG output and NG duration were evaluated as indicators of symptom control. The daily NG output was 583 ± 208 (150-1050) mL in the TDS group, which was significantly lower (P < 0.001) than that in the T group [922 ± 399 (400-1825) mL]. The need for NG tube use was also significantly shorter in the TDS group (16.7 ± 8.8 vs 27.7 ± 9.9 d, P < 0.001).
Treatment-related complications are shown in Table 4. The rate of overall complications was comparable in the TDS and T group (41.7% vs 38.2%, P = 0.770). Cholestasis, as revealed by increased bilirubin, AKP, γ-glutamyltransferase, or biliary sludge on ultrasonography, developed in 13 patients, and percutaneous transhepatic cholecystostomy (PTC) was performed in eight patients presenting with acalculous cholecystitis. The incidence of cholestasis and need for PTC were higher in the TDS group, but not significantly (P = 0.419 and 0.264, respectively). Infectious complications, including catheter-related sepsis, wound infection, and pneumonia, occurred in 15 patients. All blood culture-positive, catheter-related sepsis was cured with antibiotics. Statistical analysis revealed that there was no significant difference in infectious complications between the two groups (P = 0.677). Two patients had pneumothorax on catheter insertion.
| TPN (n = 34) | TDS (n = 36) | P value | |
| Morbidity | |||
| Cholestasis | 5 (14.7) | 8 (22.2) | 0.419 |
| Patients requiring PTC | 2 (5.9) | 5 (13.9) | 0.264 |
| Infectious complications | 8 (23.5) | 7 (19.4) | 0.677 |
| Catheter-related infections | 5 (14.7) | 4 (11.1) | 0.653 |
| Wound infection | 2 (5.9) | 1 (2.8) | 0.522 |
| Pneumonia | 1 (2.9)1 | 0 | 0.300 |
| Pneumothorax | 0 | 2 (5.5) | 0.163 |
| Overall complications | 13 (38.2) | 15 (41.7) | 0.770 |
Treatment with somatostatin and dexamethasone was well tolerated and did not cause any serious or clinical significant adverse reactions except that one patient in the intervention group complained of dry mouth.
Twelve patients were lost to follow-up (7 in the TDS group and 5 in the T group), and two patients in the T group died of relapse of primary colon cancer and gastric cancer after 12 and 18 mo of follow-up, respectively, while one in the TDS group died of cardiovascular disease (at 30 mo follow-up). Long-term follow-up data indicated that the rates of recurrence of obstruction at 1, 2 and 3 years postoperatively were similar between the two groups (Table 3). In addition, there was no significant difference in postoperative satisfaction at 1, 2 and 3 years between the two groups (Table 3).
EPSBO-OP is a rare complication after major abdominal procedures, mostly after extensive adhesiolysis. Although conservative therapy was effective in most of our cases, the patients often had to be maintained on long-term NG suction and TPN therapy before recovery of bowel function. Our results suggested that dexamethasone and somatostatin, when added to TPN, decreased the duration of NG suction and daily NG output, and shortened the duration of bowel obstruction as well as the length of hospital stay.
EPSBO-OP was first described by Fazio et al[21] and Hill et al[22] in 1983. In contrast to the common causes of EPSBO such as local adhesion, volvulus, or internal hernia, which can be managed surgically after failure of conservative treatment[23,24], OP is caused by formation of dense adhesions and severe peritoneal reaction within the early postoperative period - typically 10 d to 6-8 wk after some major procedures - especially when the bowel has fistulated. The main risk factors include extensive adhesiolysis, multiple sequential laparotomies within a short period (i.e., several days or weeks), peritonitis, and other factors causing extensive intestinal deserosalization[25,26]. The acute inflammatory reaction may involve the peritoneal surface and adherence of adjacent loops of bowel, often involving the omentum and mesenteric surfaces. These adhesions are highly vascularized, friable, and immature, thus, surgical separation is impossible. Therefore, recognition of EPSBO-OP is important to avoid serious consequences such as ECF or massive bowel resection because of re-laparotomy attempting to lyse the adhesions[27]. The adhesions are extensive, thus, the risk of closed-loop obstruction, volvulus, or strangulation is low, making conservative therapy possible[4].
Resolution of OP after prolonged TPN therapy has been reported previously. Lennard et al[8] reported two patients with ECF and OP managed with TPN for 8 and 4 mo, respectively. Selby et al[9] reported six patients with EPSBO secondary to dense and vascular benign adhesions that could not be freed by operation. The obstructions all spontaneously resolved within 2-3.5 mo on a TPN program. The authors believe that complete gastrointestinal rest allows adhesions to mature into long avascular collagen fibers in the absence of a persistent inflammatory reaction that accompanies partial or total SBO. In the current study, we also confirmed that with TPN alone, EPSBO-OP could also resolve, but it often takes a long time.
Somatostatin, or its synthetic analog octreotide, inhibits gastrointestinal secretion and release of hormones, and they have been used for treatment of SBO for over a decade, especially for malignant SBO[28]. In experimental animal models, somatostatin may be beneficial for the control of intestinal distension, inflammation and necrosis, and bacterial translocation[29,30]. Octreotide, the somatostatin analog, may ameliorate intestinal dysmotility and stasis in models of small bowel transplantation[31]. Besides its role in symptom control, somatostatin may also promote the resolution of SBO[32,33]. Zhang et al[34] have confirmed that octreotide, when combined with water-soluble radiocontrast medium, may accelerate resolution of adhesive SBO by a specific therapeutic affect. This is consistent with the findings of the current study.
Corticosteroids have long been used for their anti-inflammatory effects, which may reduce the edema and fibrin deposition associated with EPSBO-OP, thereby helping to resolve the obstruction[35]. In Japan, steroids have been used to reduce the inflammatory state of encapsulating peritoneal sclerosis, in which intraperitoneal inflammation leads to adhesive and inflammatory encapsulation of the intestinal tract, causing bowel obstructive symptoms. In a prospective cohort, 15 of 42 cases (35.7%) of encapsulating peritoneal sclerosis treated with prednisolone alone showed clinical improvement[14]. In malignant bowel obstruction, corticosteroids may reduce intestinal inflammatory edema associated with the malignant lesion, thereby aiding resolution of bowel obstruction[14]. Extensive dense inflammatory adhesions and intestinal wall edema are characteristics of EPSBO-OP, therefore, we explored the effect of corticosteroids in EPSBO-OP, and our data showed that DM, when combined with somatostatin, promoted resolution of the adhesions. Fibrin exudation and intestinal edema were most prominent in the early stage of EPSBO-OP, thus, we recommend the usage of DM immediately after diagnosis.
Cholestasis is a complication of long-term usage of somatostatin[36] and TPN. Animal models and human volunteer studies all suggest that the effect of somatostatin is associated with a pronounced decrease in bile flow, bile acid secretion, and increased bile cholesterol saturation[37-39]. In the current study, although we observed an increased incidence of cholestasis and need for PTC in patients receiving somatostatin, it did not reach statistical significance. This was possibly due to the small number of cases in our series. However, it could also be that the beneficial effect of dexamethasone on bile excretion partly counteracts the detrimental effect of somatostatin[40,41].
Increased susceptibility to infection and impaired wound healing are the main side effects of systemic corticosteroids. Trésallet et al[42] have observed that patients on steroids for > 1 mo had a higher incidence of postoperative complications, especially infections after colectomy with rectal anastomosis. We did not observe any difference in the occurrence of infection between the two groups, which was possibly because we used short-term therapy (7 d). There is currently no direct evidence that dexamethasone promotes relapse of malignancy, therefore, we did not avoid its use in tumor patients.
There were several limitations to our study. First, the diagnosis of OP was made on the basis of clinical presentation, physical examination, and medical history, and was confirmed by plain film radiography and CT, but it could not be definitively proven by laparotomy. Therefore, this may have led to the inclusion of a few cases of obstruction not caused by OP. Second, the mean operation time preceding obstruction was shorter in the TDS group, and this may have influenced the outcome. Third, the study was not blinded and the physicians and patients were aware of which therapy that each patient had received, which would have introduced some bias during evaluation.
In conclusion, our trial indicates that conservative therapy is efficient in EPSBO-OP. Administration of somatostatin and dexamethasone in addition to TPN promotes resolution of obstruction, shortens length of hospital stay, and is efficient for symptom control without increasing complications and obstruction relapse.
Early postoperative small bowel obstruction due to obliterative peritonitis (EPSBO-OP) is a rare complication after abdominal surgery, especially extensive adhesiolysis and enterocutaneous fistula. Traditionally, the only treatment for these patients was total parenteral nutrition, nasogastric tube feeding, and observation. The time to the recovery of bowel function is often long and patients often suffer from low quality of life. Methods to promote resolution and control obstruction-related symptoms are lacking.
Somatostatin or its analogs and corticosteroids are effective and safe in patients with inoperative bowel obstruction due to peritoneal carcinomatosis or encapsulating peritoneal sclerosis. Therefore, their clinical role in the management of postoperative OP warrants further investigation.
Somatostatin and dexamethasone shorten the time to obstruction resolution and length of hospital stay, and decrease nasogastric output and duration of nasogastric tube usage. They do not increase treatment-related complications and relapse of obstruction.
This study revealed that somatostatin and dexamethasone are effective in promoting resolution and controlling symptoms in patients with EPSBO-OP.
This paper provides some useful information on the management of EPSBO-OP.
P- Reviewers: Gassler N, Ho SB, Kim JH S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Li JS. Recognizing the characteristics of postoperative inflammatory small bowel obstruction. Zhongguo Shiyong Waike Zazhi. 1998;18:387-388. [Cited in This Article: ] |
| 2. | Zhu WM, Li N, Li JS. Treatment of early postoperative inflammatory small intestinal obstruction. Zhongguo Shiyong Waike Zazhi. 2002;22:219-220. [Cited in This Article: ] |
| 3. | Sajja SB, Schein M. Early postoperative small bowel obstruction. Br J Surg. 2004;91:683-691. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Helton WS, Fisichella PM. Intestinal Obstruction. ACS Surgery: Principles and Practice. New York: WebMD Inc 2004; 1-20. [Cited in This Article: ] |
| 5. | Osborn C, Fischer JE. How I do it: gastrointestinal cutaneous fistulas. J Gastrointest Surg. 2009;13:2068-2073. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Schecter WP, Hirshberg A, Chang DS, Harris HW, Napolitano LM, Wexner SD, Dudrick SJ. Enteric fistulas: principles of management. J Am Coll Surg. 2009;209:484-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 7. | Houghton SG, Medina ARDL, Sarr MG. Bowel obstruction. Maingot’s abdominal operations. 11th ed. New York: Mc Graw Hill 2007; 479-507. [Cited in This Article: ] |
| 8. | Lennard ES, Miller DG. Role of home total parenteral nutrition in management of obliterative peritonitis. JPEN J Parenter Enteral Nutr. 1981;5:154-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 9. | Selby RR, Mertz GH, Gilsdorf RB. Spontaneous resolution of intestinal obstruction while receiving home parenteral nutrition. Am J Surg. 1983;146:742-745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Mercadante S, Spoldi E, Caraceni A, Maddaloni S, Simonetti MT. Octreotide in relieving gastrointestinal symptoms due to bowel obstruction. Palliat Med. 1993;7:295-299. [PubMed] [Cited in This Article: ] |
| 11. | Ripamonti C, Mercadante S, Groff L, Zecca E, De Conno F, Casuccio A. Role of octreotide, scopolamine butylbromide, and hydration in symptom control of patients with inoperable bowel obstruction and nasogastric tubes: a prospective randomized trial. J Pain Symptom Manage. 2000;19:23-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 169] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Replogle RL, Johnson R, Gross RE. Prevention of postoperative intestinal adhesions with combined promethazine and dexamethasone therapy: experimental and clinical studies. Ann Surg. 1966;163:580-588. [PubMed] [Cited in This Article: ] |
| 13. | Kucukozkan T, Ersoy B, Uygur D, Gundogdu C. Prevention of adhesions by sodium chromoglycate, dexamethasone, saline and aprotinin after pelvic surgery. ANZ J Surg. 2004;74:1111-1115. [PubMed] [Cited in This Article: ] |
| 14. | Kawanishi H, Kawaguchi Y, Fukui H, Hara S, Imada A, Kubo H, Kin M, Nakamoto M, Ohira S, Shoji T. Encapsulating peritoneal sclerosis in Japan: a prospective, controlled, multicenter study. Am J Kidney Dis. 2004;44:729-737. [PubMed] [Cited in This Article: ] |
| 15. | Feuer DJ, Broadley KE. Corticosteroids for the resolution of malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database Syst Rev. 2000;CD001219. [PubMed] [Cited in This Article: ] |
| 16. | Pickleman J, Lee RM. The management of patients with suspected early postoperative small bowel obstruction. Ann Surg. 1989;210:216-219. [PubMed] [Cited in This Article: ] |
| 17. | Stewart RM, Page CP, Brender J, Schwesinger W, Eisenhut D. The incidence and risk of early postoperative small bowel obstruction. A cohort study. Am J Surg. 1987;154:643-647. [PubMed] [Cited in This Article: ] |
| 18. | Gong JF, Zhu WM, Li N, Li JS. Combined therapy using somatostatin analogs, steroids, and parenteral nutrition for early postoperative inflammatory small bowel obstruction. Zhonghua Waike Zazhi. 2005;20:257-258. [DOI] [Cited in This Article: ] |
| 19. | Eypasch E, Williams JI, Wood-Dauphinee S, Ure BM, Schmülling C, Neugebauer E, Troidl H. Gastrointestinal Quality of Life Index: development, validation and application of a new instrument. Br J Surg. 1995;82:216-222. [PubMed] [Cited in This Article: ] |
| 20. | Baker JW. Stitchless plication for recurring obstruction of the small bowel. Am J Surg. 1968;116:316-324. [PubMed] [Cited in This Article: ] |
| 21. | Fazio VW, Coutsoftides T, Steiger E. Factors influencing the outcome of treatment of small bowel cutaneous fistula. World J Surg. 1983;7:481-488. [PubMed] [Cited in This Article: ] |
| 22. | Hill GL. Operative strategy in the treatment of enterocutaneous fistulas. World J Surg. 1983;7:495-501. [PubMed] [Cited in This Article: ] |
| 23. | Ryan MD, Wattchow D, Walker M, Hakendorf P. Adhesional small bowel obstruction after colorectal surgery. ANZ J Surg. 2004;74:1010-1012. [PubMed] [Cited in This Article: ] |
| 24. | Srinivasa S, Kahokehr AA, Sammour T, Yu TC, Abbas SM, Hill AG. Use of statins in adhesive small bowel obstruction. J Surg Res. 2010;162:17-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Qi L, Hong ZJ, Yang L. Clinical analysis of early postoperative inflammatory small bowel obstruction. Zhonghua Waike Zazhi. 2007;6:104-106. [Cited in This Article: ] |
| 26. | Wei B, Wei HB, Guo WP, Zheng ZH, Huang Y, Hu BG, Huang JL. Diagnosis and treatment of abdominal cocoon: a report of 24 cases. Am J Surg. 2009;198:348-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Thompson JS, DiBaise JK, Iyer KR, Yeats M, Sudan DL. Postoperative short bowel syndrome. J Am Coll Surg. 2005;201:85-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Ripamonti C, Panzeri C, Groff L, Galeazzi G, Boffi R. The role of somatostatin and octreotide in bowel obstruction: pre-clinical and clinical results. Tumori. 2001;87:1-9. [PubMed] [Cited in This Article: ] |
| 29. | Mulvihill SJ, Pappas TN, Fonkalsrud EW, Debas HT. The effect of somatostatin on experimental intestinal obstruction. Ann Surg. 1988;207:169-173. [PubMed] [Cited in This Article: ] |
| 30. | Akyildiz M, Ersin S, Oymaci E, Dayangaç M, Kapkac M, Alkanat M. Effects of somatostatin analogues and vitamin C on bacterial translocation in an experimental intestinal obstruction model of rats. J Invest Surg. 2000;13:169-173. [PubMed] [Cited in This Article: ] |
| 31. | Nakada K, Ikoma A, Suzuki T, Reynolds JC, Campbell WL, Todo S, Starzl TE. Amelioration of intestinal dysmotility and stasis by octreotide early after small-bowel autotransplantation in dogs. Am J Surg. 1995;169:294-299. [PubMed] [Cited in This Article: ] |
| 32. | Kumagai K, Saikawa Y, Fukuda K, Ito R, Igarashi T, Tsuwano S, Nakamura R, Takahashi T, Takeuchi H, Kitagawa Y. Octreotide acetate successfully treated a bowel obstruction caused by peritoneally disseminated gastric cancer, thereby enabling the subsequent use of oral S-1 chemotherapy. Int J Clin Oncol. 2009;14:372-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Massacesi C, Galeazzi G. Sustained release octreotide may have a role in the treatment of malignant bowel obstruction. Palliat Med. 2006;20:715-716. [PubMed] [Cited in This Article: ] |
| 34. | Zhang Y, Gao Y, Ma Q, Dang C, Wei W, De Antoni F, Rocci R, Chen W. Randomised clinical trial investigating the effects of combined administration of octreotide and methylglucamine diatrizoate in the older persons with adhesive small bowel obstruction. Dig Liver Dis. 2006;38:188-194. [PubMed] [Cited in This Article: ] |
| 35. | Ripamonti C, De Conno F, Ventafridda V, Rossi B, Baines MJ. Management of bowel obstruction in advanced and terminal cancer patients. Ann Oncol. 1993;4:15-21. [PubMed] [Cited in This Article: ] |
| 36. | Schirmer BD, Kortz WJ, Miller RJ, Jones RS. Is somatostatin a directly acting cholestatic hormone? J Surg Res. 1985;39:237-245. [PubMed] [Cited in This Article: ] |
| 37. | Attanasio R, Mainolfi A, Grimaldi F, Cozzi R, Montini M, Carzaniga C, Grottoli S, Cortesi L, Albizzi M, Testa RM. Somatostatin analogs and gallstones: a retrospective survey on a large series of acromegalic patients. J Endocrinol Invest. 2008;31:704-710. [PubMed] [Cited in This Article: ] |
| 38. | Andersen HB, Petronijević L, Giese B, Mygind T, Burcharth F. Somatostatin reduces bile secretion and loss of bile constituents in patients with external biliary drainage. HPB Surg. 1996;9:229-233. [PubMed] [Cited in This Article: ] |
| 39. | Marteau P, Chrétien Y, Calmus Y, Parc R, Poupon R. Pharmacological effect of somatostatin on bile secretion in man. Digestion. 1989;42:16-21. [PubMed] [Cited in This Article: ] |
| 40. | Glantz A, Marschall HU, Lammert F, Mattsson LA. Intrahepatic cholestasis of pregnancy: a randomized controlled trial comparing dexamethasone and ursodeoxycholic acid. Hepatology. 2005;42:1399-1405. [PubMed] [Cited in This Article: ] |
| 41. | Hirvioja ML, Tuimala R, Vuori J. The treatment of intrahepatic cholestasis of pregnancy by dexamethasone. Br J Obstet Gynaecol. 1992;99:109-111. [PubMed] [Cited in This Article: ] |
| 42. | Trésallet C, Royer B, Godiris-Petit G, Menegaux F. Effect of systemic corticosteroids on elective left-sided colorectal resection with colorectal anastomosis. Am J Surg. 2008;195:447-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |