Published online Dec 7, 2013. doi: 10.3748/wjg.v19.i45.8408
Revised: September 4, 2013
Accepted: September 16, 2013
Published online: December 7, 2013
AIM: To evaluate the efficacy, safety and influential factors of proton pump inhibitor (PPI) treatment for non-erosive reflux disease (NERD).
METHODS: PubMed, MEDLINE, EMBASE and the Cochrane Library were searched up to April 2013 to identify eligible randomized controlled trials (RCTs) that probed into the efficacy, safety and influential factors of PPI treatment for NERD. The rates of symptomatic relief and adverse events were measured as the outcomes. After RCT selection, assessment and data collection, the pooled RRs and 95%CI were calculated. This meta-analysis was performed using the Stata 12.0 software (Stata Corporation, College Station, Texas, United States). The level of evidence was estimated by the Grading of Recommendations Assessment, Development and Evaluation system.
RESULTS: Seventeen RCTs including 6072 patients met the inclusion criteria. The results of the meta-analysis showed that PPI treatment was significantly superior to H2 receptor antagonists (H2RA) treatment (RR = 1.629, 95%CI: 1.422-1.867, P = 0.000) and placebo (RR = 1.903, 95%CI: 1.573-2.302, P = 0.000) for the symptomatic relief of NERD. However, there were no obvious differences between PPI and H2RA (RR = 0.928, 95%CI: 0.776-1.110, P = 0.414) or PPI and the placebo (RR = 1.000, 95%CI: 0.896-1.116, P = 0.997) regarding the rate of adverse events. The overall rate of symptomatic relief of PPI against NERD was 51.4% (95%CI: 0.433-0.595, P = 0.000), and relief was influenced by hiatal hernia (P = 0.030). The adverse rate of PPI against NERD was 21.0% (95%CI: 0.152-0.208, P = 0.000), and was affected by hiatal hernia (P = 0.081) and drinking (P = 0.053).
CONCLUSION: PPI overmatched H2RA on symptomatic relief rate but not on adverse rate for NERD. Its relief rate and adverse rate were influenced by hiatal hernia and drinking.
Core tip: As a kind of powerful and effective acid-suppressive drugs, proton pump inhibitor (PPI) has been used for patients with non-erosive reflux disease (NERD), but its efficacy, safety and their influential factors are inconclusive. We performed this systematic review and meta-analysis of randomized controlled trials to assess its efficacy, safety and influential factors. Based on the results of the meta-analysis, we conclude that PPI has a higher symptomatic relief rate and roughly the same adverse rate for NERD. Hiatal hernia and drinking could influence symptomatic relief rate and adverse rate of PPI on NERD.
- Citation: Zhang JX, Ji MY, Song J, Lei HB, Qiu S, Wang J, Ai MH, Wang J, Lv XG, Yang ZR, Dong WG. Proton pump inhibitor for non-erosive reflux disease: A meta-analysis. World J Gastroenterol 2013; 19(45): 8408-8419
- URL: https://www.wjgnet.com/1007-9327/full/v19/i45/8408.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i45.8408
Non-erosive reflux disease (NERD) is a heterogeneous group of disorders, which present with the typical gastroesophageal reflux symptoms of heartburn, regurgitation or both in the absence of visible esophageal injury upon endoscopy[1-3]. Patients with NERD are more likely to be female, young, thin, and without hiatal hernias[4-6], and over time, the regurgitation of gastric juice associated with NERD can have significant and comparable negative effects on their quality of life that correlate with heartburn severity[7-9]. To improve these patients’ quality of life, provide a rapid relief of symptoms and reduce the severity and number of recurrent episodes[10-12], acid-suppressive drugs have been used to combat NERD.
Proton pump inhibitors (PPI) are a type of acid-suppressive drugs that inhibit the secretion of gastric acid by restraining the exchange of H+-K+[13,14]. Due to their powerful inhibition of the secretion of gastric acid, PPIs have been widely used to treat gastroesophageal diseases that result from too much acid, including gastroesophageal reflux disease, gastritis and gastric and duodenal ulcers[15-18]. However, the efficacy, safety and influential factors of PPI use remain inconclusive, especially for NERD[19,20].
Although two papers[21,22] have previously discussed the efficacy and influential factors of PPI use against NERD, neither paper used randomized controlled trials (RCTs) as the source of their data or used H2 receptor antagonists (H2RA) or placebos as control groups. Meanwhile, the clinical safety of PPIs was not addressed by the authors of these two papers. In view of the importance of understanding their clinical implications, we determined that the quality of the previous two papers was insufficient and performed the present meta-analysis.
We conducted a computer-aided search for RCTs which probed into the efficacy, safety and influential factors of PPI for NERD. Source databases were PubMed (1966 to April 2013), the Cochrane Library (1997 to April 2013), MEDLINE (1966 to April 2013) and EMBASE (1985 to April 2013). The medical subject headings which were used in retrieving citation were: non-erosive reflux disease or NERD, proton pump inhibitors or PPI or esomeprazole or pantoprazole or omeprazole or rabeprazole or lansoprazole. We also searched the references in retrieved articles manually in order to prevent missing relevant publications.
The titles and abstracts were independently screened by two reviewers (Zhang JX and Song J), and studies were chosen for the meta-analysis if they fit the following criteria: (1) randomized controlled trials; (2) comparing PPI with other acid-suppressive drugs or placebo; and (3) probing into the efficacy, safety and influential factors of PPI on the symptomatic relief of NERD. We did not consider the restriction on language of publication. Exclusion criteria were: (1) no human subjects in the study; (2) without control group; (3) comparing a PPI with another one; (4) incomplete outcome data; (5) selective reporting; and (6) duplicate publication.
Independently, three reviewers (Qiu S, Ai MH and Wang J) extracted data including the following items: first author, year of publication, country, type of publication, study duration, age, gender, medication duration, drug dose, follow-up time, methods of treatment, H. pylori infection and primary outcomes. Based on the adequate sequence generation, allocation concealment, blinding, incomplete outcome data addressed, free of selective reporting, free from baseline imbalance, sample size calculation and free from sources of funding bias, the risk of bias was evaluated in detail. Each quality component was judged as high, unclear, or low. On the basis of each separate component, the quality of the trials was assessed. When difference appeared, a forth reviewer (Lei HB) joined in the discussion.
We treated the rates of symptomatic relief of PPI vs placebo and PPI vs H2RA as the primary endpoints and the rates of adverse events as the secondary endpoints. Meanwhile, factors influencing rates of symptomatic relief and adverse events of PPI against NERD were analyzed. The RRs, to summary statistics in meta-analysis, were strongly recommended for dichotomous data. So we used Stata12.0 to calculate RR for the rates of symptomatic relief and the rates of adverse events in this meta-analysis. When the P value was less than 0.05, it was considered significant. The data was pooled according to the Mantel-Haenszel fixed-effects model and the DerSimonian and Laird random-effects model. The differences were shown as pooled RRs and 95%CI between different groups. The statistical heterogeneity among trials was assessed by the χ2 test and I2 test. The percentage of the variability in the estimates of effect, caused by heterogeneity but not chance, was described by I2 test. When the values were greater than 50%, it was considered having substantial heterogeneity. If there was no statistically significant heterogeneity, the fixed-effects model was chosen. According to the drug dose and therapeutic duration, subgroup analysis was performed.
We assessed the risks of bias according to assessment of study quality in Cochrane Handbook 4.2.2. Egger’s test and Begg’s test were used to check the publication bias, and P < 0.05 indicated that there was a risk of bias.
Sensitivity analysis was performed to identify the studies which influence the result obviously.
Meta-regression analysis was performed to study the relationship between covariates and the outcomes and to find the source of heterogeneity.
Grade system was applied to assess the quality of these outcomes.
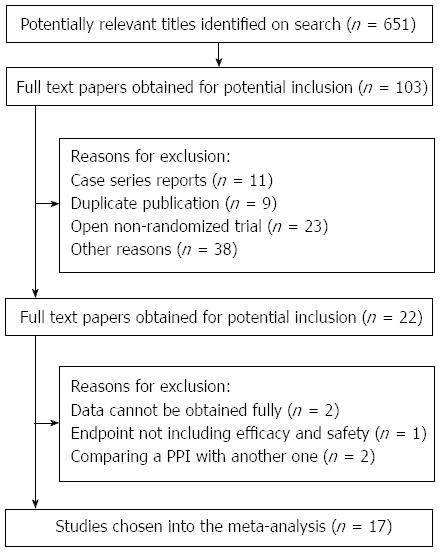
Following the searching strategy, we initially acquired 651 studies. Having discarded the studies of repetitive publication and that did not meet the criteria apparently, and after reading the titles and abstracts, there were 51 studies left. To search and read the full text, 34 studies which were not RCTs or without control groups were abandoned and 17 RCTs[23-39] were left finally. The screening process of studies is shown in Figure 1.
For the 17 RCTs, 5 were single-center studies and 12 were multi-center studies. In the RCTs, there were 6072 patients with 3937 patients in the combination group and the 2135 patients in PPI alone group. The details of these studies are listed in Table 1.
| First author | Country | Year | Study design | Arms of treatment | Age (yr) | Gender (M/F) | n | BMI | Helicobacter pylori infection | Hiatal hernia | Smokers | Alcohol users | Therapeutic duration | Adverse events | Effective |
| Talley | United | 2002 | RCT | Esomeprazole 20 mg | 48.0 | 135/158 | 293 | - | 90 | 89 | - | - | 6 mo | 5 | 101 (34.6) |
| et al[23] | Kingdom | Esomeprazole 40 mg | 48.4 | 135/147 | 282 | - | 101 | 92 | - | - | 6 | 84 (29.7) | |||
| placebo | 48.2 | 58/88 | 146 | - | 57 | 39 | - | - | 2 | 30 (17.8) | |||||
| Juul-Hansen | Norway | 2009 | RCT | Lansoprazole 15 mg | 47.5 | 11/21 | 32 | 26.4 | 6 | 10 | 8 | - | 6 mo | 2 | 28 (87.5) |
| et al[24] | Ranitidine 75 mg | 48.0 | 12/19 | 31 | 25.2 | 6 | 6 | 8 | - | 4 | 14 (45.2) | ||||
| Kinoshita | Japan | 2011 | RCT | Rabeprazole 5 mg | 46.3 | 38/55 | 93 | 22.4 | 35 | - | - | - | 4 wk | 32 | 36 (35.0) |
| et al[25] | Rabeprazole 10 mg | 46.8 | 50/51 | 101 | 23.2 | 42 | - | - | - | 37 | 44 (44.0) | ||||
| placebo | 49.7 | 40/51 | 91 | 23.2 | 44 | - | - | - | 32 | 19 (21.0) | |||||
| Kobeissy | Lebanon | 2012 | RCT | Rabeprazole 20 mg | 45.4 | 16/28 | 44 | - | - | - | - | - | 4 wk | - | 20 (45.5) |
| et al[26] | Ranitidine 300 mg | 45.1 | 16/23 | 39 | - | - | - | - | - | - | 17 (43.6) | ||||
| Talley | Australia | 2001 | RCT | Esomeprazole 20 mg | 49.0 | 94/76 | 170 | - | 64 | 65 | - | - | 6 mo | 11 | 146 (85.0) |
| et al[27] | placebo | 49.0 | 98/74 | 172 | - | 57 | 75 | - | - | 13 | 83 (48.0) | ||||
| Fass | United States | 2009 | RCT | Dexlansoprazole 30 mg | 47.6 | 84/231 | 315 | 29.0 | 95 | - | 72 | 162 | 4 wk | 6 | 173 (54.9) |
| et al[28] | Dexlansoprazole 60 mg | 47.5 | 106/209 | 315 | 29.6 | 90 | - | 57 | 181 | 8 | 157 (50.0) | ||||
| placebo | 47.6 | 84/233 | 317 | 29.1 | 89 | - | 52 | 182 | 1 | 59 (18.5) | |||||
| Fujiwara | Japan | 2005 | RCT | Omeprazole 20 mg | 55.0 | 20/30 | 50 | 22.8 | 22 | 14 | 6 | 18 | 4 wk | - | 43 (86.0) |
| et al[29] | Famotidine 20 mg | 56.6 | 23/25 | 48 | 22.2 | 22 | 11 | 11 | 17 | - | 36 (75.0) | ||||
| Nakamura | Japan | 2010 | RCT | Omeprazole 20 mg | 51.4 | 8/9 | 17 | - | 5 | 9 | - | - | 8 wk | 0 | 13 (75.0) |
| et al[30] | Roxitidine 75 mg | 48.6 | 9/7 | 16 | - | 7 | 8 | - | - | 0 | 11 (70.6) | ||||
| Miner | United States | 2002 | RCT | Rabeprazole 10 mg | 44.4 | 28/37 | 65 | 30.9 | 17 | - | 22 | 29 | 4 wk | 14 | 19 (29.3) |
| et al[31] | Rabeprazole 20 mg | 45.5 | 25/33 | 68 | 30.3 | 24 | - | 21 | 26 | 17 | 19 (28.3) | ||||
| placebo | 46.1 | 24/26 | 70 | 30.2 | 28 | - | 22 | 30 | 18 | 2 (3.4) | |||||
| Armstrong | Canada | 2001 | RCT | Pantoprazole 40 mg | 47.1 | 57/49 | 106 | - | 16 | - | 66 | 71 | 4 wk | 32 | 41 (52.8) |
| et al[32] | Nizatidine 150 mg | 47.6 | 51/51 | 102 | - | 20 | - | 64 | 67 | 39 | 33 (42.9) | ||||
| Lind | Sweden | 1999 | RCT | Omeprazole 10 mg | 52.0 | 53/86 | 139 | - | - | 76 | 24 | 88 | 4 wk | - | 116 (83.5) |
| et al[33] | Omeprazole 20 mg | 51.0 | 65/77 | 142 | - | - | 72 | 43 | 85 | - | 99 (69.7) | ||||
| placebo | 48.0 | 61/82 | 143 | - | - | 74 | 57 | 97 | - | 80 (55.9) | |||||
| Richter | United States | 2000 | RCT | Lansoprazole 15 mg | 44.9 | 115/161 | 276 | - | 66 | - | 75 | 132 | 8 wk | - | 37 (13.4) |
| et al[34] | Lansoprazole 30 mg | 45.1 | 122/155 | 277 | - | 66 | - | 88 | 129 | - | 97 (35.0) | ||||
| Ranitidine 150 mg | 45.2 | 108/170 | 278 | - | 63 | - | 69 | 126 | - | 64 (23.0) | |||||
| placebo | 46.3 | 30/37 | 67 | - | 9 | - | 19 | 35 | - | 10 (14.7) | |||||
| Bytzer | Denmark | 2004 | RCT | Rabeprazole 10 mg | 47.0 | 123/156 | 279 | 26.0 | 100 | - | - | - | 6 mo | 113 | 241 (86.4) |
| et al[12] | placebo | 48.0 | 57/82 | 139 | 27.0 | 52 | - | - | - | 47 | 94 (67.6) | ||||
| Kahrilas | United States | 2009 | RCT | Rabeprazole 20 mg | 43.1 | 40/89 | 129 | 29.3 | 42 | - | 35 | 58 | 4 wk | - | 42 (32.4) |
| et al[35] | placebo | 44.0 | 45/87 | 132 | 30.0 | 40 | - | 36 | 51 | - | 5 (3.8) | ||||
| Lind | Sweden | 1997 | RCT | Omeprazole 10 mg | 49.0 | 89/110 | 199 | - | - | - | 60 | 133 | 4 wk | 6 | 98 (49.2) |
| et al[33] | Omeprazole 20 mg | 50.0 | 66/139 | 205 | - | - | - | 46 | 121 | 11 | 125 (61.0) | ||||
| placebo | 51.0 | 51/54 | 105 | - | - | - | 22 | 64 | 7 | 25 (23.8) | |||||
| Uemura | Japan | 2008 | RCT | Omeprazole 10 mg | 44.4 | 47/49 | 96 | - | 37 | 5 | - | - | 4 wk | 12 | 44 (45.8) |
| et al[36] | Omeprazole 20 mg | 43.8 | 53/40 | 93 | - | 49 | 9 | - | - | 22 | 43 (46.2) | ||||
| placebo | 42.4 | 53/49 | 92 | - | 32 | 2 | - | - | 17 | 22 (23.9) | |||||
| Talley | Australia | 2002 | RCT | Pantoprazole 20 mg | 53.0 | 78/76 | 154 | 28.9 | - | - | 65 | 28 | 6 mo | 86 | 109 (71.0) |
| et al[27] | Ranitidine 150 mg | 52.0 | 83/70 | 153 | 28.4 | - | - | 61 | 30 | 83 | 86 (56.0) |
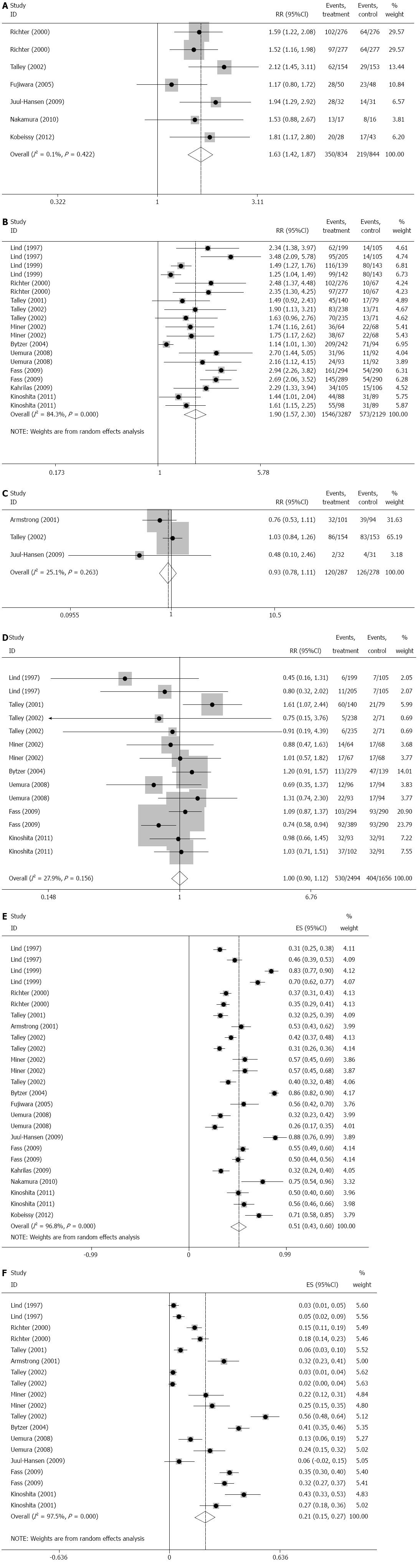
Seven studies[24,26,29,30,32,34,39], which involved 1882 patients, compared PPI with H2RA on the rate of symptomatic relief of NERD. There are 935 patients who received PPI and 947 patients who received H2RA. Heterogeneity analysis showed that there was obviously statistical heterogeneity among these studies (I2 = 42.4%, P = 0.096). Sensitivity analysis indicated that one study[32] influenced the result apparently, and after excluding this study, the heterogeneity disappeared (I2 = 0.1%, P = 0.422). The result showed that PPI was significantly superior to H2RA on the rate of symptomatic relief of NERD (RR = 1.629, 95%CI: 1.422-1.867, P = 0.000), (Figure 2A).
In the subgroup analysis of short duration (PPI 158/372, placebo 112/384, I2 = 0%, P = 0.640), PPI advanced over H2RA (RR = 1.521, 95%CI: 1.303-1.775, P = 0.000). In the subgroup analysis of long duration (PPI 90/186, placebo 43/184, I2 = 0%, P = 0.737), similar result was found (RR = 2.063, 95%CI: 1.544-2.756, P = 0.000).
In the subgroup analysis of low dose (PPI 130/308, placebo 78/307, I2 = 0%, P = 0.422), PPI significantly overmatched H2RA (RR = 1.656, 95%CI: 1.320-2.078, P = 0.000). In the subgroup analysis of high dose (PPI 220/526, placebo 141/537, I2 = 23.5%, P = 0.365), PPI was also superior to H2RA (RR = 1.614, 95%CI: 1.361-1.914, P = 0.000).
In the subgroup analysis of lansoprazole (PPI 227/585, placebo 141/584, I2 = 0%, P = 0.603), PPI advanced over H2RA (RR = 1.866, 95%CI: 1.435-2.448, P = 0.000). But compared with groups of omeprazole (PPI 41/67, placebo 31/64, I2 = 0%, P = 0.434), there were no statistical differences (P = 0.149).
There were 11 studies[23,25,27,28,31,33-38] which compared PPI with placebo on the rate of symptomatic relief of NERD. In the 5416 patients of the 11 trials, there are 3287 patients who received PPI and 2129 patients received placebo. Heterogeneity analysis showed that there was obviously statistical heterogeneity among these studies (I2 = 84.3%, P = 0.000). Sensitivity analysis did not find studies that influenced the result obviously. The result showed that PPI was significantly superior to placebo on the rate of symptomatic relief of NERD (RR = 1.903, 95%CI: 1.573-2.302, P = 0.000), (Figure 2B).
In the subgroup analysis of long duration (PPI 407/855, placebo 114/315, I2 = 65.4%, P = 0.034), PPI advanced over placebo (RR = 1.442, 95%CI: 1.034-2.010, P = 0.031). In short duration (PPI 1139/2432, placebo 459/1241, I2 = 78.6%, P = 0.000), similar result was also found (RR = 2.029, 95%CI: 1.665-2.473, P = 0.000).
In the subgroup analysis of high dose (PPI 486/1098, placebo 131/718, I2 = 0%, P = 0.506), PPI significantly overmatched placebo (RR = 2.664, 95%CI: 2.251-3.154, P = 0.000). In low dose (PPI 1060/2189, placebo 442/1411, I2 = 75.1%, P = 0.000), PPI was significantly superior to placebo (RR = 1.726; 95%CI: 1.451-2.054, P = 0.000).
In the subgroup analysis of lansoprazole (PPI 505/1136, placebo 128/714, I2 = 0%, P = 0.879), pantoprazole (PPI 252/649, placebo 88/320, I2 = 0%, P = 0.844), omeprazole (PPI 427/874, placebo 210/680, I2 = 81.4%, P = 0.000) and rabeprazole (PPI 317/478, placebo 130/336, I2 = 81.3%, P = 0.001), PPI advanced over placebo (P = 0.000).
Three studies[24,32,39], which involved 565 patients, compared PPI with H2RA on the rate of adverse events of NERD. There were 287 patients who received PPI and 278 patients who received H2RA. Because there was no obviously statistical heterogeneity among these studies (I2 = 25.1%, P = 0.263), fixed-effects model was chosen to perform the meta-analysis. The result showed that there was no significantly difference between PPI and H2RA on the rate of adverse events of NERD (RR = 0.928; 95%CI: 0.776-1.110, P = 0.414, Figure 2C).
There were eight studies[23,25,27,28,31,36] which compared PPI with placebo on the rate of adverse events of NERD. Among the 4150 patients, 2494 patients received PPI and 1656 patients received placebo. Because there was no obviously statistical heterogeneity among these studies (I2 = 27.9%, P = 0.156), fixed-effects model was chosen to perform the meta-analysis. The result showed that there was no significant difference between PPI and placebo on the rate of adverse events of NERD (RR = 1.000; 95%CI: 0.896-1.116, P = 0.997), (Figure 2D).
In the subgroup analysis of long duration (PPI 184/892, placebo 72/360, I2 = 8.5%, P = 0.364), there was no significant difference between PPI and placebo (RR = 0.921, 95%CI: 0.812-1.046, P = 0.206). In short duration (PPI 346/1602, placebo 332/1296, I2 = 0%, P = 0.565), PPI was significantly superior to placebo (RR = 1.290, 95%CI: 1.032-1.613, P = 0.025).
In the subgroup analysis of high dose (PPI 316/1661, placebo 252/1068, I2 = 45.6%, P = 0.075), there was no obvious difference between PPI and placebo (RR = 0.999, 95%CI: 0.868-1.150, P = 0.988). In low dose (PPI 214/833, placebo 152/588, I2 = 2.9%, P = 0.398), no significant difference was found either (OR = 1.002, 95%CI: 0.841-1.195, P = 0.979).
In the subgroup analysis of lansoprazole (PPI 308/962, placebo 233/719, I2 = 75.4%, P = 0.017), pantoprazole (PPI 80/668, placebo 68/224, I2 = 0%, P = 0.981), omeprazole (PPI 51/593, placebo 48/398, I2 = 23.5%, P = 0.270) and rabeprazole (PPI 31/131, placebo 34/136, I2 = 0%, P = 0.732), the significant difference was also not found (P > 0.05).
All the 17 studies[23-39] provided the data of the efficacy of PPI against NERD and its influential factors. Heterogeneity analysis showed that there was obviously statistical heterogeneity among these studies (I2 = 96.8%, P = 0.000). The result showed that the overall rate of symptomatic relief of PPI against NERD was 51.4% (95%CI: 0.433-0.595, P = 0.000), (Figure 2E).
In the subgroup analysis of long duration, the effective rate of PPI against NERD was 51.4% (95%CI: 0.433-0.595, P = 0.000). In short duration, the rate was 51.5% (95%CI: 0.432-0.598, P = 0.000).
In the subgroup analysis of high dose, the effective rate of PPI against NERD was 48.4% (95%CI: 0.404-0.564, P = 0.000). In low dose, the rate was 56.3% (95%CI: 0.395-0.732, P = 0.000).
In the subgroup analysis of lansoprazole, the effective rate of PPI against NERD was 52.1% (95%CI: 0.392-0.650, P = 0.000). In that of pantoprazole, omeprazole and rabeprazole, the effective rate were 44.7% (95%CI: 0.369-0.526, P = 0.000), 52.1% (95%CI: 0.355-0.688, P = 0.000) and 60.8% (95%CI: 0.367-0.849, P = 0.000), respectively.
Univariate meta-regression analysis found that the rate of hiatal hernia (P = 0.030) was associated with the rate of symptomatic relief of PPI against NERD, but not with others.
Twelve studies[23-25,27,28,31,32,34,37-39] provided the data of the rate of adverse events of PPI against NERD and its influential factors. Heterogeneity analysis showed that there was obviously statistical heterogeneity among these studies (I2 = 97.5%, P = 0.000). Sensitivity analysis indicated that no study influenced the result apparently. The result showed that the adverse rate of PPI against NERD was 21.0% (95%CI: 0.152-0.208, P = 0.000), (Figure 2F).
In the subgroup analysis of long duration, the adverse rate of PPI against NERD was 18.0% (95%CI: 0.094-0.265, P = 0.000). In short duration, the rate was 23.3% (95%CI: 0.145-0.322, P = 0.000).
In the subgroup analysis of high dose, the adverse rate of PPI against NERD was 21.1% (95%CI: 0.152-0.268, P = 0.000). In low dose, the rate was 20.8% (95%CI: 0.100-0.317, P = 0.000).
In the subgroup analysis of lansoprazole, the adverse rate of PPI against NERD was 21.5% (95%CI: 0.121-0.309, P = 0.000). In that of pantoprazole, omeprazole and rabeprazole, the effective rate respectively were 26.2% (95%CI: 0.150-0.375, P = 0.000), 9.8% (95%CI: 0.036-0.161, P = 0.002) and 29.5% (95%CI: 0.165-0.426, P = 0.000).
Univariate meta-regression analysis found that the rate of hiatal hernia (P = 0.081) and drinking (P = 0.053) were associated with the rate of adverse events of PPI against NERD, but not with the other factors (Table 2).
| Factors | Efficacy | Adverse rate | ||
| Coefficient | P value | Coefficient | P value | |
| Age | 0.0177315 | 0.170 | 0.0075041 | 0.665 |
| Gender | -0.2213605 | 0.186 | 0.0209630 | 0.894 |
| BMI | -0.0127987 | 0.484 | -0.0044024 | 0.808 |
| n | -0.0005643 | 0.154 | -0.0001338 | 0.733 |
| Helicobacter pylori infection | -0.6007750 | 0.217 | 0.1326016 | 0.736 |
| Hiatal hernia | 0.9702707 | 0.030 | -0.4392244 | 0.081 |
| Smoking | -0.2591453 | 0.528 | 0.5030517 | 0.245 |
| Drinking | 0.3296374 | 0.303 | -0.6776039 | 0.053 |
| Therapeutic duration | 0.0008200 | 0.857 | -0.0015192 | 0.722 |
| Dose | -0.0026090 | 0.414 | 0.0003684 | 0.897 |
In the analysis of PPI vs H2RA on the rate of symptomatic relief, sensitivity analysis indicated that one study[32] influenced the result apparently, and after excluding the this study, the heterogeneity disappeared (I2 = 0.1%, P = 0.422). And in other analysis, there was no study which influenced the results.
Three studies[26,28,32] performed adequate sequence generation with the others unclear. No study carried out allocation concealment. Two studies[24,26] were open-label trials without blinding of participants and personnel and 11 studies[23,25,27,28,31-36,39] mentioned blinding of participants and personnel. All the studies had complete data, without selective reporting and other bias. According to the Egger’s test and Begg’s test, we did not find obvious publication bias in the outcome of PPI vs H2RA on the rate of symptomatic relief (Egger’s test: P = 0.711 and Begg’s test: P = 0.646), PPI vs H2RA on the rate of adverse events (Egger’s test: P = 1.000 and Begg’s test: P = 0.374) and PPI vs placebo on the rate of adverse events (Egger’s test: P = 0.125 and Begg’s test: P = 0.552). But in the outcome of PPI vs placebo on the rate of symptomatic relief, the potential publication bias may exist (Egger’s test: P = 0.010 and Begg’s test: P = 0.013). A language bias, inflated estimates by a flawed methodologic design in smaller studies, and/or a lack of publication of small trials with opposite results may be the causes.
Following the classification of the Grading of Recommendations Assessment, Development and Evaluation, the quality of evidences and their causes are shown in Table 3.
| Outcome | Study design | Risk of bias | Inconsistecy | Indirectness | Imprecision | Publication bias | Quality of evidence |
| PPI vs H2RA on the rate of symptomatic relief | RCT | Serious1 | No | No | No | Serious4 | Low |
| Long-duration subgroup | RCT | Serious2 | No | No | No | Serious4 | Low |
| Short-duration subgroup | RCT | Serious2 | No | No | No | Serious4 | Low |
| High-dose subgroup | RCT | Serious2 | No | No | No | Serious4 | Low |
| Lose-dose subgroup | RCT | Serious2 | No | No | No | Serious4 | Low |
| PPI vs placebo on the rate of symptomatic relief | RCT | No | Series3 | No | No | No | Moderate |
| Long-duration subgroup | RCT | No | Series3 | No | No | No | Moderate |
| Short-duration subgroup | RCT | No | No | No | No | No | High |
| High-dose subgroup | RCT | No | No | No | No | No | High |
| Lose-dose subgroup | RCT | No | Series3 | No | No | No | Moderate |
| PPI vs H2RA on the rate of adverse events | RCT | Serious2 | No | No | No | Serious4 | Low |
| PPI vs placebo on the rate of adverse events | RCT | No | Series3 | No | No | No | Moderate |
| Long-duration subgroup | RCT | No | No | No | No | No | High |
| Short-duration subgroup | RCT | No | Series3 | No | No | No | Moderate |
| High-dose subgroup | RCT | No | Series3 | No | No | No | Moderate |
| Lose-dose subgroup | RCT | No | No | No | No | No | High |
| Overall efficacy of PPI against NERD | RCT | Serious1 | Series3 | No | No | No | Low |
| Long-duration subgroup | RCT | Serious2 | Series3 | No | No | No | Low |
| Short-duration subgroup | RCT | Serious2 | No | No | No | No | Moderate |
| High-dose subgroup | RCT | Serious2 | Series3 | No | No | No | Low |
| Lose-dose subgroup | RCT | Serious2 | No | No | No | No | Moderate |
| Overall safety of PPI against NERD | RCT | Serious2 | Series3 | No | No | Serious4 | Low |
| Long-duration subgroup | RCT | Serious2 | Series3 | No | No | Serious4 | Low |
| Short-duration subgroup | RCT | No | No | No | No | Serious4 | High |
| High-dose subgroup | RCT | No | Series3 | No | No | Serious4 | Moderate |
| Lose-dose subgroup | RCT | Serious2 | No | No | No | Serious4 | Moderate |
PPIs have been widely used to treat NERD, but their efficacy, safety and influential factors are unclear. Our meta-analysis, including 17 well-designed randomized controlled trials, 12 of which were multi-center and 5 of which were single-center, had systematically and comprehensively evaluated the evidence concerning the efficacy, safety and influential factors of PPIs against NERD.
The first major finding revealed by this comprehensive approach was that the activity of PPIs is obviously superior to that of H2RA in its efficacy and safety against NERD. Because heartburn, the main symptom of patients with NERD, results from erosion due to gastric acid reflux into the esophagus, acid-suppressive drugs, including PPI and H2RA, have been deemed effective treatments for NERD[40,41]. After a meal, gastrin secretion stimulates the release of histamine by enterochromaffin-like cells, which binds to histamine H2 receptors, leading to acid release via the hydrogen potassium ATPase (H+-K+-ATPase) pump[42]. Compared to the mechanism of H2RA, which acts against one of the three histamine-H2 receptors, PPI acts against the H+-K+-ATPase[43]. To control for the influences of different dose and therapeutic duration, we performed a subgroup analysis. This analysis showed that PPI treatment against NERD was superior to H2RA and placebo regardless of the dose or duration. However, only after short durations was PPI treatment safer than placebo.
The second major finding of this meta-analysis was that the overall rate of symptomatic relief of PPI against NERD was 51.4%; this value was influenced by the presence of a hiatal hernia. Compared with the approximate 50% symptomatic relief rate of PPI against ERD[44,45], the 51.4% rate of PPI against NERD is fairly high. PPIs with a high dose, long duration and from a new generation should be more effective than those with a low dose, short duration and from an older generation; however, according to our subgroup analysis, there were no obvious differences among different doses, durations and PPI types. PPI enacts its role by binding to the binding sites of the saturable enzyme H+-K+-ATPase; therefore, an excessively high blood concentration of PPI is not only unable to increase but even decreases the acid suppression effect of the enzyme. Univariate meta-regression analysis found that the rate of hiatal hernia was associated with the rate of the symptomatic relief of PPI use against NERD. One role of the gastroesophageal junction is to minimize gastroesophageal reflux; hiatal hernias, which are protrusions (or herniations) of the upper part of the stomach into the thorax through a tear or weakness in the diaphragm, can cause reflux and reduce the clear effects of the esophagus[46]. Due to their effects on gastroesophageal reflux and the normal function of the esophagus, the presence of hiatal hernias may influence the symptomatic relief rate of PPIs against NERD.
The third major finding of this meta-analysis was that the adverse rate of PPI treatment against NERD was 21.0%; this value was affected by hiatal hernia and drinking. PPI use was not, however, without shortcomings. Primary adverse events, typically in the order of 1%-5%, included headache, diarrhea, constipation, nausea, and rash[47]. Long-term PPI use was able to cause diminished acid secretion and reduced somatostatin release, resulting in enterochromaffin-like cell hyperplasia and hypergastrinemia[48,49]. As indicated by univariate meta-regression analysis, the adverse rate of PPI use for NERD was influenced by hiatal hernia and drinking. The mechanism through which hiatal hernia influences the adverse rate of PPI for NERD is uncertain, but the reason might be that hiatal hernias cause reflux, stimulating the nausea-inducing receptors in the esophageal and throat, as well as other adverse events. In addition, the metabolism of PPI generates two different CYP isoforms in the liver, which are responsible for the majority of their biotransformation due to their susceptibility to ethyl alcohol (CYP2C19 and CYP3A4)[50-52]. Thus, as drinking increases the blood concentration of ethyl alcohol, adverse events due to the reduced biotransformation of CYP2C19 and CYP3A4 and an increased blood concentration of PPI may arise.
There are a few shortcomings in our meta-analysis that should be mentioned. First, the analytical results are influenced by the reviewers, although we attempted to overcome this drawback. Second, a few differences may exist due to the various assessments of the efficacy and safety of PPI against NERD. Third, the evaluation index resulted from subjective feelings, which may influence the authenticity of these studies.
In conclusion, our meta-analysis showed that PPI is more effective than H2RA or placebo for the treatment of NERD. However, there was no significant difference between the safeties of PPI and H2RA or placebo. In addition, the effective rate of PPI for NERD was associated with hiatal hernia, while the adverse rate was associated with hiatal hernia and drinking. In the clinic, it is necessary to choose a PPI with a suitable dose, therapeutic duration and type for different NERD patients. More multi-center, high-quality randomized controlled trials with larger samples and longer term of follow-up visits are desirable.
Patients with non-erosive reflux disease (NERD) suffer from heartburn due to gastric acid in the reflux content. Acid-suppressive drugs, especially proton pump inhibitor (PPI), have been used widely to manage NERD.
Though PPI has been used for patients with NERD for years, however, sufficient and convictive evidences concerning its efficacy and safety are lacking and whether its efficacy and safety are influenced by other factors remains unclear.
The meta-analysis and systematic review was conducted according to Cochrane Handbook. The rates of symptomatic relief of PPI vs placebo and PPI vs H2 receptor antagonists (H2RA) were treated as the primary endpoint and the rates of adverse events as the secondary endpoint. Meanwhile, factors influencing rates of symptomatic relief and adverse events of PPI against NERD are analyzed.
This meta-analysis indicated that PPI overmatched H2RA on symptomatic relief rate but not on adverse rate for NERD. The rate of symptomatic relief of PPI against NERD was influenced by hiatal hernia and the adverse rate was affected by hiatal hernia and drinking.
This is a well written, sufficiently interesting original article in which the authors reviewed the efficacy, safety and their influential factors of PPI against NERD.
P- Reviewers: Jadallah KA, Shimatan T, Tosetti C S- Editor: Gou SX L- Editor: Ma JY E- Editor: Wang CH
| 1. | Chey WD. Endoscopy-negative reflux disease: concepts and clinical practice. Am J Med. 2004;117 Suppl 5A:36S-43S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Wang C, Hunt RH. Precise role of acid in non-erosive reflux disease. Digestion. 2008;78 Suppl 1:31-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Minatsuki C, Yamamichi N, Shimamoto T, Kakimoto H, Takahashi Y, Fujishiro M, Sakaguchi Y, Nakayama C, Konno-Shimizu M, Matsuda R. Background factors of reflux esophagitis and non-erosive reflux disease: a cross-sectional study of 10,837 subjects in Japan. PLoS One. 2013;8:e69891. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 4. | Moayyedi P, Hunt R, Armstrong D, Lei Y, Bukoski M, White R. The impact of intensifying acid suppression on sleep disturbance related to gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther. 2013;37:730-737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Shida H, Sakai Y, Hamada H, Takayama T. The daily response for proton pump inhibitor treatment in Japanese reflux esophagitis and non-erosive reflux disease. J Clin Biochem Nutr. 2013;52:76-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Ke MY. How to differentiate non-erosive reflux disease from functional heartburn. J Dig Dis. 2012;13:605-608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Bardhan KD, Müller-Lissner S, Bigard MA, Bianchi Porro G, Ponce J, Hosie J, Scott M, Weir DG, Gillon KR, Peacock RA. Symptomatic gastro-oesophageal reflux disease: double blind controlled study of intermittent treatment with omeprazole or ranitidine. The European Study Group. BMJ. 1999;318:502-507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 93] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Galmiche JP, Bruley des Varannes S. Endoluminal therapies for gastro-oesophageal reflux disease. Lancet. 2003;361:1119-1121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Mancini V, Ribolsi M, Gentile M, de’Angelis G, Bizzarri B, Lindley KJ, Cucchiara S, Cicala M, Borrelli O. Oesophageal mucosal intercellular space diameter and reflux pattern in childhood erosive and non-erosive reflux disease. Dig Liver Dis. 2012;44:981-987. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Fock KM, Talley N, Hunt R, Fass R, Nandurkar S, Lam SK, Goh KL, Sollano J. Report of the Asia-Pacific consensus on the management of gastroesophageal reflux disease. J Gastroenterol Hepatol. 2004;19:357-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Fock KM, Teo EK, Ang TL, Chua TS, Ng TM, Tan YL. Rabeprazole vs esomeprazole in non-erosive gastro-esophageal reflux disease: a randomized, double-blind study in urban Asia. World J Gastroenterol. 2005;11:3091-3098. [PubMed] [Cited in This Article: ] |
| 12. | Bytzer P, van Zanten SV, Mattsson H, Wernersson B. Partial symptom-response to proton pump inhibitors in patients with non-erosive reflux disease or reflux oesophagitis - a post hoc analysis of 5796 patients. Aliment Pharmacol Ther. 2012;36:635-643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Linsky A, Hermos JA, Lawler EV, Rudolph JL. Proton pump inhibitor discontinuation in long-term care. J Am Geriatr Soc. 2011;59:1658-1664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Wilhelm SM, Rjater RG, Kale-Pradhan PB. Perils and pitfalls of long-term effects of proton pump inhibitors. Expert Rev Clin Pharmacol. 2013;6:443-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 15. | Fujiwara Y, Takahashi S, Arakawa T, Sollano JD, Zhu Q, Kachintorn U, Rani AA, Hahm KB, Joh T, Kinoshita Y. A 2008 questionnaire-based survey of gastroesophageal reflux disease and related diseases by physicians in East Asian countries. Digestion. 2009;80:119-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Bruley des Varannes S, Coudsy B, Waechter S, Delemos B, Xiang J, Lococo J, Ducrotté P. On-demand proton pump inhibitory treatment in overweight/obese patients with gastroesophageal reflux disease: are there pharmacodynamic arguments for using higher doses? Digestion. 2013;88:56-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Chitapanarux T, Praisontarangkul OA, Lertprasertsuke N. An open-labeled study of rebamipide treatment in chronic gastritis patients with dyspeptic symptoms refractory to proton pump inhibitors. Dig Dis Sci. 2008;53:2896-2903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Takeuchi T, Umegaki E, Takeuchi N, Yoda Y, Kojima Y, Tokioka S, Higuchi K. Strategies for peptic ulcer healing after 1 week proton pump inhibitor-based triple Helicobacter pylori eradication therapy in Japanese patients: differences of gastric ulcers and duodenal ulcers. J Clin Biochem Nutr. 2012;51:189-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Scarpignato C. Poor effectiveness of proton pump inhibitors in non-erosive reflux disease: the truth in the end! Neurogastroenterol Motil. 2012;24:697-704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Woodland P, Sifrim D. Management of gastro-oesophageal reflux disease symptoms that do not respond to proton pump inhibitors. Curr Opin Gastroenterol. 2013;29:431-436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Weijenborg PW, Cremonini F, Smout AJ, Bredenoord AJ. PPI therapy is equally effective in well-defined non-erosive reflux disease and in reflux esophagitis: a meta-analysis. Neurogastroenterol Motil. 2012;24:747-757, e350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 22. | Hiyama T, Matsuo K, Urabe Y, Fukuhara T, Tanaka S, Yoshihara M, Haruma K, Chayama K. Meta-analysis used to identify factors associated with the effectiveness of proton pump inhibitors against non-erosive reflux disease. J Gastroenterol Hepatol. 2009;24:1326-1332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Talley NJ, Venables TL, Green JR, Armstrong D, O’Kane KP, Giaffer M, Bardhan KD, Carlsson RG, Chen S, Hasselgren GS. Esomeprazole 40 mg and 20 mg is efficacious in the long-term management of patients with endoscopy-negative gastro-oesophageal reflux disease: a placebo-controlled trial of on-demand therapy for 6 months. Eur J Gastroenterol Hepatol. 2002;14:857-863. [PubMed] [Cited in This Article: ] |
| 24. | Juul-Hansen P, Rydning A. On-demand requirements of patients with endoscopy-negative gastro-oesophageal reflux disease: H2-blocker vs. proton pump inhibitor. Aliment Pharmacol Ther. 2009;29:207-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Kinoshita Y, Ashida K, Hongo M; Japan Rabeprazole Study Group for NERD. Randomised clinical trial: a multicentre, double-blind, placebo-controlled study on the efficacy and safety of rabeprazole 5 mg or 10 mg once daily in patients with non-erosive reflux disease. Aliment Pharmacol Ther. 2011;33:213-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Kobeissy AA, Hashash JG, Jamali FR, Skoury AM, Haddad R, El-Samad S, Ladki R, Aswad R, Soweid AM. A randomized open-label trial of on-demand rabeprazole vs ranitidine for patients with non-erosive reflux disease. World J Gastroenterol. 2012;18:2390-2395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Talley NJ, Lauritsen K, Tunturi-Hihnala H, Lind T, Moum B, Bang C, Schulz T, Omland TM, Delle M, Junghard O. Esomeprazole 20 mg maintains symptom control in endoscopy-negative gastro-oesophageal reflux disease: a controlled trial of ‘on-demand’ therapy for 6 months. Aliment Pharmacol Ther. 2001;15:347-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 140] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 28. | Fass R, Chey WD, Zakko SF, Andhivarothai N, Palmer RN, Perez MC, Atkinson SN. Clinical trial: the effects of the proton pump inhibitor dexlansoprazole MR on daytime and nighttime heartburn in patients with non-erosive reflux disease. Aliment Pharmacol Ther. 2009;29:1261-1272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Fujiwara Y, Higuchi K, Nebiki H, Chono S, Uno H, Kitada K, Satoh H, Nakagawa K, Kobayashi K, Tominaga K. Famotidine vs. omeprazole: a prospective randomized multicentre trial to determine efficacy in non-erosive gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2005;21 Suppl 2:10-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Nakamura K, Akiho H, Ochiai T, Motomura Y, Higuchi N, Okamoto R, Matsui N, Yasuda D, Akahoshi K, Kabemura T. Randomized controlled trial: roxatidine vs omeprazole for non-erosive reflux disease. Hepatogastroenterology. 2010;57:497-500. [PubMed] [Cited in This Article: ] |
| 31. | Miner P, Orr W, Filippone J, Jokubaitis L, Sloan S. Rabeprazole in nonerosive gastroesophageal reflux disease: a randomized placebo-controlled trial. Am J Gastroenterol. 2002;97:1332-1339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 117] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | Armstrong D, Paré P, Pericak D, Pyzyk M. Symptom relief in gastroesophageal reflux disease: a randomized, controlled comparison of pantoprazole and nizatidine in a mixed patient population with erosive esophagitis or endoscopy-negative reflux disease. Am J Gastroenterol. 2001;96:2849-2857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 16] [Reference Citation Analysis (0)] |
| 33. | Lind T, Havelund T, Lundell L, Glise H, Lauritsen K, Pedersen SA, Anker-Hansen O, Stubberöd A, Eriksson G, Carlsson R. On demand therapy with omeprazole for the long-term management of patients with heartburn without oesophagitis--a placebo-controlled randomized trial. Aliment Pharmacol Ther. 1999;13:907-914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 140] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Richter JE, Campbell DR, Kahrilas PJ, Huang B, Fludas C. Lansoprazole compared with ranitidine for the treatment of nonerosive gastroesophageal reflux disease. Arch Intern Med. 2000;160:1803-1809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 105] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 35. | Bytzer P, Blum A, De Herdt D, Dubois D; Trial Investigators. Six-month trial of on-demand rabeprazole 10 mg maintains symptom relief in patients with non-erosive reflux disease. Aliment Pharmacol Ther. 2004;20:181-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Kahrilas PJ, Miner P, Johanson J, Mao L, Jokubaitis L, Sloan S. Efficacy of rabeprazole in the treatment of symptomatic gastroesophageal reflux disease. Dig Dis Sci. 2005;50:2009-2018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Lind T, Havelund T, Carlsson R, Anker-Hansen O, Glise H, Hernqvist H, Junghard O, Lauritsen K, Lundell L, Pedersen SA. Heartburn without oesophagitis: efficacy of omeprazole therapy and features determining therapeutic response. Scand J Gastroenterol. 1997;32:974-979. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 292] [Cited by in F6Publishing: 288] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 38. | Uemura N, Inokuchi H, Serizawa H, Chikama T, Yamauchi M, Tsuru T, Umezu T, Urata T, Yurino N, Tanabe S. Efficacy and safety of omeprazole in Japanese patients with nonerosive reflux disease. J Gastroenterol. 2008;43:670-678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Talley NJ, Moore MG, Sprogis A, Katelaris P. Randomised controlled trial of pantoprazole versus ranitidine for the treatment of uninvestigated heartburn in primary care. Med J Aust. 2002;177:423-427. [PubMed] [Cited in This Article: ] |
| 40. | Pace F, Casini V, Pallotta S. Heterogeneity of endoscopy negative heartburn: epidemiology and natural history. World J Gastroenterol. 2008;14:5233-5236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 41. | Katz PO, Castell DO, Levine D. Esomeprazole resolves chronic heartburn in patients without erosive oesophagitis. Aliment Pharmacol Ther. 2003;18:875-882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Shin JM, Munson K, Vagin O, Sachs G. The gastric HK-ATPase: structure, function, and inhibition. Pflugers Arch. 2009;457:609-622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 160] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 43. | Ward RM, Kearns GL. Proton pump inhibitors in pediatrics: mechanism of action, pharmacokinetics, pharmacogenetics, and pharmacodynamics. Paediatr Drugs. 2013;15:119-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 44. | Kahrilas PJ, Falk GW, Johnson DA, Schmitt C, Collins DW, Whipple J, D’Amico D, Hamelin B, Joelsson B. Esomeprazole improves healing and symptom resolution as compared with omeprazole in reflux oesophagitis patients: a randomized controlled trial. The Esomeprazole Study Investigators. Aliment Pharmacol Ther. 2000;14:1249-1258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 256] [Cited by in F6Publishing: 235] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 45. | Farley A, Wruble LD, Humphries TJ. Rabeprazole versus ranitidine for the treatment of erosive gastroesophageal reflux disease: a double-blind, randomized clinical trial. Raberprazole Study Group. Am J Gastroenterol. 2000;95:1894-1899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Hata M, Shiono M, Sekino H, Furukawa H, Sezai A, Iida M, Yoshitake I, Hattori T, Wakui S, Taoka M. Efficacy of a proton pump inhibitor given in the early postoperative period to relieve symptoms of hiatal hernia after open heart surgery. Surg Today. 2006;36:131-134. [PubMed] [Cited in This Article: ] |
| 47. | Chubineh S, Birk J. Proton pump inhibitors: the good, the bad, and the unwanted. South Med J. 2012;105:613-618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 48. | Laine L, Ahnen D, McClain C, Solcia E, Walsh JH. Review article: potential gastrointestinal effects of long-term acid suppression with proton pump inhibitors. Aliment Pharmacol Ther. 2000;14:651-668. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 182] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 49. | di Mario F, Cavallaro LG. Non-invasive tests in gastric diseases. Dig Liver Dis. 2008;40:523-530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 50. | Li Y, Zhang W, Guo D, Zhou G, Zhou H, Xiao Z. Pharmacokinetics of the new proton pump inhibitor ilaprazole in Chinese healthy subjects in relation to CYP3A5 and CYP2C19 genotypes. Clin Chim Acta. 2008;391:60-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 51. | Perera MA. The missing linkage: what pharmacogenetic associations are left to find in CYP3A? Expert Opin Drug Metab Toxicol. 2010;6:17-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 52. | Furuta T, Shirai N, Sugimoto M, Nakamura A, Hishida A, Ishizaki T. Influence of CYP2C19 pharmacogenetic polymorphism on proton pump inhibitor-based therapies. Drug Metab Pharmacokinet. 2005;20:153-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 191] [Article Influence: 10.1] [Reference Citation Analysis (0)] |