Published online Jan 28, 2013. doi: 10.3748/wjg.v19.i4.511
Revised: September 28, 2012
Accepted: October 22, 2012
Published online: January 28, 2013
AIM: To investigate endoscopic ultrasound (EUS) for predicting depth of mucosal invasion and to analyze outcomes following endoscopic and transduodenal resection.
METHODS: Records of 111 patients seen at our institution from November 1999 to July 2011 with the post-operative pathological diagnosis of benign ampullary and duodenal adenomas were reviewed. Records of patients who underwent preoperative EUS for diagnostic purposes were identified. The accuracy of EUS in predicting the absence of muscular invasion was assessed by comparing EUS reports to the final surgical pathological results. In addition, the incidence of the post-operative complications over a period of 30 d and the subsequent long-term outcome (recurrence) over a period of 30 mo associated with endoscopic and transduodenal surgical resection was recorded, compared and analyzed.
RESULTS: Among 111 patients with benign ampullary and duodenal adenomas, 47 underwent preoperative EUS for 29 peri-ampullary lesions and 18 duodenal lesions. In addition, computed tomography was performed in 18 patients, endoscopic retrograde cholangio-pancreatography in 10 patients and esophagogastroduodenoscopy in 22 patients. There were 43 patients with sporadic adenomas and 4 patients with familial adenomatous polyposis (FAP)/other polyposis syndromes. In 38 (81%, P < 0.05) patients, EUS reliably identified absence of submucosal and muscularis invasion. In 4 cases, EUS underestimated submucosal invasion that was proven by pathology. In the other 5 patients, EUS predicted muscularis invasion which could not be demonstrated in the resected specimen. EUS predicted tumor muscularis invasion with a specificity of 88% and negative predictive value of 90% (P < 0.05). Types of resection performed included endoscopic resection in 22 cases, partial duodenectomy in 9 cases, transduodenal ampullectomy with sphincteroplasty in 10 cases and pancreaticoduodenectomy in 6 cases. The main post-operative final pathological results included villous adenoma (n = 5), adenoma (n = 8), tubulovillous adenoma (n = 10), tubular adenoma (n = 20) and hyperplastic polyp (n = 2). Among the 47 patients who underwent resection, 8 (17%, 5 of which corresponded to surgical resection) developed post-procedural complications which included retroperitoneal hematoma, intra-abdominal abscess, wound infection, delayed gastric emptying and prolonged ileus. After median follow-up of 20 mo there were 6 local recurrences (13%, median follow-up = 20 mo) 4 of which were in patients with FAP.
CONCLUSION: EUS accurately predicts the depth of mucosal invasion in suspected benign ampullary and duodenal adenomas. These patients can safely undergo endoscopic or local resection.
- Citation: Azih LC, Broussard BL, Phadnis MA, Heslin MJ, Eloubeidi MA, Varadarajulu S, Arnoletti JP. Endoscopic ultrasound evaluation in the surgical treatment of duodenal and peri-ampullary adenomas. World J Gastroenterol 2013; 19(4): 511-515
- URL: https://www.wjgnet.com/1007-9327/full/v19/i4/511.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i4.511
Duodenal tumors often pose diagnostic and therapeutic challenges in their management as they arise in close proximity to biliary and pancreatic structures[1]. Villous tumors of the duodenum was first described by Perry in 1893 as a broad-based cauliflower-like mass that he referred to as a duodenal papilloma and in a 1981 review, only 73 cases had been reported by Komorowski et al[2]. Although tumors that arise in the duodenum are not typically common (only 1% of gastrointestinal tumors) a significant fraction of them (at least 25%) are adenomas at the time of presentation. Treatment of ampullary adenoma is complicated by difficult preoperative staging, malignant potential and a high recurrence rate[3]. Various techniques are advocated for their management ranging from simple excision to the ampullary tumor and the contiguous duodenal mucosa to wide resection of the mass including the papilla and adjacent duodenal, ductal and pancreatic tissue[4]. Despite their benign nature peri-ampullary and duodenal adenomas remain a therapeutic challenge because of their potential for malignant transformation. Surgical resection of duodenal tumors can be particularly difficult because of their location in the retroperitoneal space in contact with normal pancreatic gland entailing a high risk of postoperative morbidity and mortality[5] . The complex anatomy of the ampullary region and the difficulty of accurately diagnosing and staging via imaging and endoscopy makes the management of these tumors controversial and require multi-disciplinary teams[6]. While these tumors can typically arise anywhere along the duodenum, they predominately appear in the first or second portion with tumors in the 3rd and 4th portions being less frequent but posing more difficulty in endoscopic detection[1]. Accurate preoperative histological diagnosis and staging of these tumors is therefore often difficult and inconclusive leading to controversy over the ideal management of treating these lesions. Transduodenal local excision (TDE), endoscopic snare excision or pancreatoduodenectomy (PD) are valid options for resection of these tumors[6]. Halstead first reported TDE of an ampullary mass in 1899[7]. In 1935 Whipple was the first to perform en bloc removal of the entire duodenum with the head of the pancreas in 1935 a procedure later refined to a one-stage procedure called PD in 1940 by Whipple[8]. Some studies have reported that the incidence of malignancy occurring in duodenal or ampullary tumors vary widely with some reports ranging from 35% to 60% and report that while the region of the ampulla can be accessible for endoscopic biopsy procedure, that there is a high incidence of false negative results for carcinoma, ranging from 25% to 60%[7]. In 1990, a collective series of 78 Japanese patients reported that the biopsy diagnosis of adenoma does not rule out the possibility of deeper carcinoma in ampullary tumors[9]. Another case report in 1992 found EUS as an emerging useful adjuvant to the preoperative evaluation of patients for potential local resection due to its ability to accurately diagnose duodenal adenomas and therefore usefulness in treatment planning[10]. Based on these earlier reported high incidence of malignancy and unreliability of preoperative endoscopic diagnosis, optimal operative management of these tumors remains controversial[7]. We performed a retrospective review of patients with suspected benign duodenal tumors who underwent preoperative EUS to determine the accuracy of this technique in predicting the absence of muscular invasion and also to analyze outcomes associated with endoscopic and transduodenal surgical resection at our institution.
Records of 111 patients evaluated and treated at the University of Alabama Birmingham from November 1999 to July 2011 with the post-operative pathological diagnosis of benign ampullary and duodenal adenomas were identified and reviewed retrospectively. There were 43 patients with sporadic adenomas and 4 patients with familial adenomatous polyposis (FAP)/other polyposis syndromes. Patients were 55% women and average age was 63 years old (Table 1.)
| Age, yr, mean ± SD (range) | 63.4 ± 12.63 (25-90) |
| Sex | 45% male; 55% female |
| Types of resection | Endoscopic (22); pancreaticoduodenectomy (6); transduodenal (10); partial duodenectomy (9) |
| Tumor location | Periampullary (29); duodenal (18) |
| Post operative final path | Villous adenoma (5); tubulovillous adenoma (10); tubular adenoma (20); hyperplastic polyp (2); chronic inflammation (1); adenoma (8); Reactive epithelial changes of normal small bowel mucosa (1) |
| EUS prediction | Absence of muscularis invasion (n = 5, P < 0.05); underestimated submucosal invasion (n = 4, P < 0.05); Accurately predicted depth of tumor invasion (n = 38, P < 0.05) |
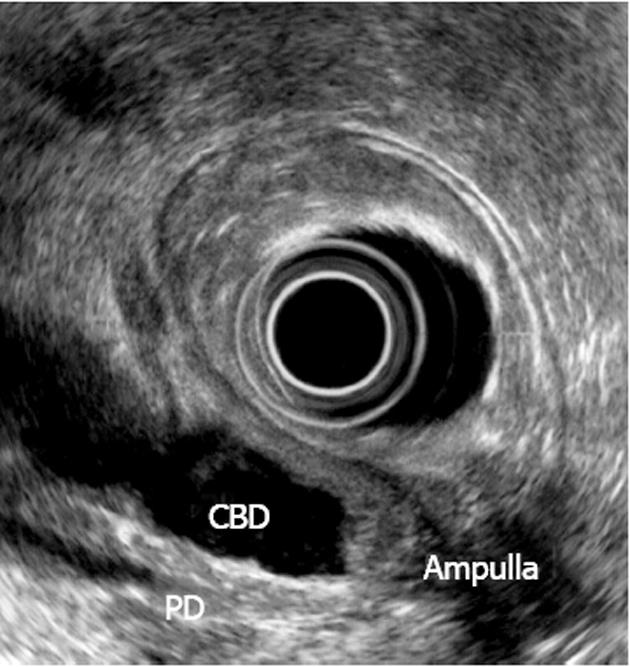
Among 111 patients examined, 47 underwent preoperative EUS for diagnostic purposes (Figure 1). In addition, computed tomography (CT) was performed in 18 patients, endoscopic retrograde cholangiopancreatography in 10 patients and esophagogastroduodenoscopy in 22 patients.
The accuracy of EUS in predicting duodenal adenoma absence of muscular invasion was assessed by comparing EUS reports and comparing it to final surgical pathological results. In addition, the incidence of post-operative complications over a period of 30 d and subsequent long-term outcome (recurrence) over a period of 30 mo associated with endoscopic and transduodenal surgical resection was recorded, compared and analyzed.
Twenty-nine patients had peri-ampullary tumors defined by immediate proximity (within 2 cm) to the major duodenal papilla. The remaining 18 patients had adenomas elsewhere in the duodenum as described in Table 1.
Resection of the analyzed adenomas was performed endoscopically in 22 cases. Partial duodenectomy was performed in 9 cases, TDE with ampullectomy with sphincteroplasty in 10 cases and pancreaticoduodenectomy in 6 cases.
Postoperative final pathology results included villous adenoma (n = 5), adenoma (n = 8), tubulovillous adenoma (n = 10), tubular adenoma (n = 20) and hyperplastic polyp (n = 2), chronic inflammation (n = 1), reactive changes of normal small bowel mucosa (n = 1).
After median follow-up of 20 mo, there were 6 local recurrences (13%) 4 of which developed in patients with FAP (Table 2). Among the 47 patients who underwent resection, 8 (17%, 5 of which corresponded to surgical resection) developed post-procedural complications, which included retroperitoneal hematoma, intra-abdominal abscess, wound infection, delayed gastric emptying and prolonged ileus. EUS reliably identified absence of submucosal and muscularis invasion in 38 (81%) (P < 0.05) patients. There were 4 patients in whom EUS under-estimated invasion of the deep layers which was subsequently demonstrated in the final pathological analysis. In 5 cases (3 of which underwent pancreaticoduodenectomy, 1 underwent endoscopic resection, and 1 underwent transduodenal resection) EUS over-estimated submucosal invasion as this feature could not be proven in the resected specimen. Overall analysis therefore demonstrated that EUS predicted tumor muscularis invasion with a specificity of 88% and negative predictive value (NPV) of 90% (P < 0.05).
The characterization of benign duodenal and peri-ampullary tumors, offers a diagnostic challenge to reliably distinguish adenomas from malignant lesions and render the possibility of transduodenal resection. While duodenal tumors are not common accounting for less than 1% of total gastrointestinal tumors and 25% of them are benign, the precise characterization continues to present a diagnostic challenge secondary to the complexity of the involved anatomical region. The surgical management of duodenal tumors is also challenging largely due to their close relationship with the pancreatic and biliary ducts and their deep location[5] .
The incidence of malignancy occurring in duodenal or ampullary tumors varies widely with false negative results for carcinoma as high as 60%[7] in the peri-ampullary region.
PD is the treatment of choice for invasive malignancies arising in the ampullary region and duodenum but its indication for treatment of benign peri-ampullary lesions is less clear. Several studies indicate that TDE exhibits less mortality and morbidity than PD[11]. Ampullectomy has been recommended as the procedure of choice to resect benign lesions smaller than 3 cm. TDE is therefore an organ-preserving operation with low morbidity but careful attention to the complex nature of the anatomy of the peri-ampullary region must be given to maximize its chances of success[11] . EUS is reportedly helpful in identifying non-invasive lesions suitable for local resection, but no preoperative test has been proven accurate enough to substitute for clinical judgment and intra-operative pathological confirmation[12]. In a study of local resection for ampullary tumors, the findings recommend local ampullary resection as an acceptable treatment in benign and selected premalignant and malignant ampullary lesions with a low threshold for conversion to PD when appropriate[13]. Another study of 63 patients proposed PD for even benign lesions because two patients in their series had to undergo repeat operations (PD) 4 and 22 years later for stage IV disease[14]. Local resection has been shown to be a viable alternative to PD in patients with benign tumors or as a palliative procedure in malignant cases with severe co morbidities where radical resection carries unacceptable surgical risk[15]. A review of 19 cases of villous tumors of the duodenum, suggests that some small benign ampullary villous adenomas or those with carcinoma in situ can be excised locally but prefer PD in the fit patient for better local control both of extensive benign lesions and cancers without distant metastases[16]. Other proponents of local resection confirm that benign duodenal villous tumors can be managed successfully by local submucosal excision[17]. In selected patients, endoscopic mucosal resection of superficial neoplastic lesions is associated with low morbidity when compared to surgery[18]. Pre-operative evaluation of these tumors to assess tumor depth is therefore paramount when planning the optimal therapeutic approach for resection of benign duodenal and peri-ampullary tumors. EUS has emerged as a useful technique in assessing the depth of invasion and is often employed when planning therapeutic approach. EUS images of tumors of the duodenal papilla correspond well to the final histological findings and report EUS as a reliable procedure for determining the extent of tumors in this location[19]. Comparison of EUS with other imaging modalities such as conventional sonography, CT, and angiography proved it to be the most effective method for local staging of pancreatic and ampullary cancers[20]. EUS can readily detect the presence of an ampullary or duodenal tumor in 96% of cases and reliably characterizes malignant lesions[21,22].
Consistent with prior publications, 16 of the 18 duodenal tumors in our study had tumors in the first or second portion of the duodenum (Table 1). Considering the above stated advantages, EUS is often employed at our institution when planning therapeutic approach to these tumors (Figure 1). Multiple studies have shown that EUS is superior to CT, magnetic resonance imaging, and transabdominal US in local peri-ampullary staging[23,24]. Previous studies evaluating the role of endoscopic resection of ampullary adenomas have presented it as a reasonable alternative to transduodenal surgical excision but long term follow-up data are needed to evaluate pre-operative staging accuracy and recurrence rates[25,26] .
Our retrospective review shows that patients with peri-ampullary tumors and EUS showing absence of muscularis invasion can safely undergo transduodenal ampullectomy with sphincteroplasty and that this procedure results in satisfactory long-term outcomes. Muscularis invasion and pancreatic duct dilatation are features of malignant neoplasms that can be safely ruled out by EUS. Our findings further support endoscopic resection and TDE as safe treatment modalities for benign duodenal adenomas that avoid morbidity associated with PD and are associated with satisfactory long-term outcomes.
We conclude that EUS can accurately predict depth of mucosal invasion in the preoperative evaluation of suspected peri-ampullary and duodenal adenomas. These patients can safely undergo endoscopic or local resection with acceptable local control rates sparing the need for more extensive operations.
Management of benign duodenal and peri-ampullary adenomas is controversial as to the best management strategy. Some proponents of a more radical approach advocate a more aggressive pancreaticoduodenectomy while other proponents are in favor of a less invasive local resection.
Endoscopic ultrasound (EUS) has emerged as a useful modality in the preoperative evaluation of these lesions and is used at our institution to guide management planning.
Duodenal tumors often pose diagnostic and therapeutic challenges in their management as they arise in close proximity to biliary and pancreatic structures and EUS has been shown to be a useful means of preoperative evaluation of these lesions to guide with the management planning. In one study, the sensitivity of EUS in the diagnosis of benign tumors was reported as 92%. The management of these benign tumors continues to be controversial posing a challenge to the surgeon, endoscopist and patients.
Based on their results, EUS can accurately predict depth of mucosal invasion in 81% (P < 0.05) of benign ampullary and duodenal adenomas with a specificity of 88% (P < 0.05) and negative predictive value of 90% (P < 0.05). These patients can safely undergo endoscopic or local resection with acceptable local control rates sparing the need for more extensive operations.
Endoscopic ultrasound is an imaging modality that is used mostly in the upper digestive tract and in the respiratory system. It involves the insertion of a probe into a hollow organ and using ultrasound it is able to obtain images of internal organs that are in the chest, and abdomen and aids in visualizing these structures as well as blood vessels adjacent to them using the doppler imaging. Gastroenterologists with advanced training typically perform the procedure. Transduodenal ampullectomy with sphincteroplasty is a local surgical excision procedure that is less invasive than the standard pancreaticoduodenectomy which is a radical resection typically used for more invasive lesions.
This is a unique collection of cases establishing the value of endoscopic ultrasound in the management of duodenal and peri-ampullary adenomas and also reviewing the suitability and the results of the various possible operative procedures. With attention to minor details, it is an important paper and well worth publishing.
P- Reviewer Tovey FI S- Editor Gou SX L- Editor A E- Editor Xiong L
| 1. | Yan JQ, Peng CH, Yang WP, Ding JZ, Zhou GW, Ma D, Li HW. Surgical management of benign duodenal tumours. ANZ J Surg. 2010;80:526-530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Chappuis CW, Divincenti FC, Cohn I. Villous tumors of the duodenum. Ann Surg. 1989;209:593-598; discussion 593-598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Cahen DL, Fockens P, de Wit LT, Offerhaus GJ, Obertop H, Gouma DJ. Local resection or pancreaticoduodenectomy for villous adenoma of the ampulla of Vater diagnosed before operation. Br J Surg. 1997;84:948-951. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 96] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Treitschke F, Beger HG. Local resection of benign periampullary tumors. Ann Oncol. 1999;10 Suppl 4:212-214. [PubMed] [Cited in This Article: ] |
| 5. | Cavallini M, Cavaniglia D, Felicioni F, Vitale V, Pilozzi E, Ziparo V. Large periampullary villous tumor of the duodenum. J Hepatobiliary Pancreat Surg. 2007;14:526-528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Paramythiotis D, Kleeff J, Wirtz M, Friess H, Büchler MW. Still any role for transduodenal local excision in tumors of the papilla of Vater? J Hepatobiliary Pancreat Surg. 2004;11:239-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Posner S, Colletti L, Knol J, Mulholland M, Eckhauser F. Safety and long-term efficacy of transduodenal excision for tumors of the ampulla of Vater. Surgery. 2000;128:694-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 79] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Howe JR, Klimstra DS, Moccia RD, Conlon KC, Brennan MF. Factors predictive of survival in ampullary carcinoma. Ann Surg. 1998;228:87-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 237] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 9. | Yamaguchi K, Enjoji M, Kitamura K. Endoscopic biopsy has limited accuracy in diagnosis of ampullary tumors. Gastrointest Endosc. 1990;36:588-592. [PubMed] [Cited in This Article: ] |
| 10. | Tio TL, Sie LH, Verbeek PC, Dé Wit LT, Tytgat GN. Endosonography in diagnosing and staging duodenal villous adenoma. Gut. 1992;33:567-568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Sakorafas GH, Sarr MG. Local excision of periampullary villous tumours of the duodenum. Eur J Surg Oncol. 1999;25:90-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Rattner DW, Fernandez-del Castillo C, Brugge WR, Warshaw AL. Defining the criteria for local resection of ampullary neoplasms. Arch Surg. 1996;131:366-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 13. | Asbun HJ, Rossi RL, Munson JL. Local resection for ampullary tumors. Is there a place for it? Arch Surg. 1993;128:515-520. [PubMed] [Cited in This Article: ] |
| 14. | Chareton B, Coiffic J, Landen S, Bardaxoglou E, Campion JP, Launois B. Diagnosis and therapy for ampullary tumors: 63 cases. World J Surg. 1996;20:707-712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Farouk M, Niotis M, Branum GD, Cotton PB, Meyers WC. Indications for and the technique of local resection of tumors of the papilla of Vater. Arch Surg. 1991;126:650-652. [PubMed] [Cited in This Article: ] |
| 16. | Ryan DP, Schapiro RH, Warshaw AL. Villous tumors of the duodenum. Ann Surg. 1986;203:301-306. [PubMed] [Cited in This Article: ] |
| 17. | Bjork KJ, Davis CJ, Nagorney DM, Mucha P. Duodenal villous tumors. Arch Surg. 1990;125:961-965. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 46] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Ahmad NA, Kochman ML, Long WB, Furth EE, Ginsberg GG. Efficacy, safety, and clinical outcomes of endoscopic mucosal resection: a study of 101 cases. Gastrointest Endosc. 2002;55:390-396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 273] [Cited by in F6Publishing: 269] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 19. | Yasuda K, Mukai H, Cho E, Nakajima M, Kawai K. The use of endoscopic ultrasonography in the diagnosis and staging of carcinoma of the papilla of Vater. Endoscopy. 1988;20 Suppl 1:218-222. [PubMed] [Cited in This Article: ] |
| 20. | Rösch T, Braig C, Gain T, Feuerbach S, Siewert JR, Schusdziarra V, Classen M. Staging of pancreatic and ampullary carcinoma by endoscopic ultrasonography. Comparison with conventional sonography, computed tomography, and angiography. Gastroenterology. 1992;102:188-199. [PubMed] [Cited in This Article: ] |
| 21. | Will U, Bosseckert H, Meyer F. Correlation of endoscopic ultrasonography (EUS) for differential diagnostics between inflammatory and neoplastic lesions of the papilla of Vater and the peripapillary region with results of histologic investigation. Ultraschall Med. 2008;29:275-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Kalantzis N, Laoudi F, Kallimanis G, Gabriel P, Farmakis N. The role of endoscopic ultrasonography in diagnosis of benign lesions of the upper GI tract. Eur J Surg Oncol. 1993;19:449-454. [PubMed] [Cited in This Article: ] |
| 23. | Chini P, Draganov PV. Diagnosis and management of ampullary adenoma: The expanding role of endoscopy. World J Gastrointest Endosc. 2011;3:241-247. [PubMed] [Cited in This Article: ] |
| 24. | Chen CH, Tseng LJ, Yang CC, Yeh YH. Preoperative evaluation of periampullary tumors by endoscopic sonography, transabdominal sonography, and computed tomography. J Clin Ultrasound. 2001;29:313-321. [PubMed] [Cited in This Article: ] |
| 25. | Hernandez LV, Catalano MF. Endoscopic papillectomy. Curr Opin Gastroenterol. 2008;24:617-622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Bohra AK, McKie L, Diamond T. Transduodenal excision of ampullary tumours. Ulster Med J. 2002;71:121-127. [PubMed] [Cited in This Article: ] |