Published online Oct 21, 2013. doi: 10.3748/wjg.v19.i39.6548
Revised: July 21, 2013
Accepted: August 17, 2013
Published online: October 21, 2013
Processing time: 210 Days and 21.4 Hours
The Authors summarize problems, criticisms but also advantages and indications regarding the recent surgical proposal of associating liver partition and portal vein ligation (PVL) for staged hepatectomy (ALPPS) for the surgical management of colorectal liver metastases. Looking at published data, the technique, when compared with other traditional and well established methods such as PVL/portal vein embolisation (PVE), seems to give real advantages in terms of volumetric gain of future liver remnant. However, major concerns are raised in the literature and some questions remain unanswered, preliminary experiences seem to be promising. The method has been adopted all over the world over the last 2 years, even if oncological long-term results remain unknown, and benefit for patients is questionable. No prospective studies comparing traditional methods (PVE, PVL or classical 2 staged hepatectomy) with ALPPS are available to date. Technical reinterpretations of the original method were also proposed in order to enhance feasability and increase safety of the technique. More data about morbidity and mortality are also expected. The real role of ALPPS is, to date, still to be established. Large clinical studies, even if, for ethical reasons, in well selected cohorts of patients, are expected to better define the indications for this new surgical strategy.
Core tip: The recent publication by Regensburg’s Group on the new technical possibility of associating liver partition and portal vein ligation for staged hepatectomy for the surgical managing of bilateral colorectal liver metastases, generated a great debate and a burst of publications about preliminary experiences from many groups all over the world. As one of the first groups in Germany to adopt this technique, in the present article we clarify some aspects of our experience in the light of published data and raised concerns.
- Citation: Donati M, Stavrou GA, Oldhafer KJ. Current position of ALPPS in the surgical landscape of CRLM treatment proposals. World J Gastroenterol 2013; 19(39): 6548-6554
- URL: https://www.wjgnet.com/1007-9327/full/v19/i39/6548.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i39.6548
The progressive enlargement of surgical indications to colo-rectal liver metastasis (CRLM) resection of the last 20 years has led to a redefinition of resectability criteria[1]. Nowadays no limits due to number of lesions and location are of value as in the past[2]. The main problem for resectability criteria of CRLM is due to volume of future liver remnant[3], in fact, postoperative liver failure is one of the biggest risk and a significant complication after extended hepatectomy[4]. Some techniques were established such as portal vein ligation (PVL) and portal vein embolisation (PVE) with well known advantages and limits. Also 2 stage combined strategies were developed to overcome resectability problems due to bilobar location of metastases and two-staged hepatectomy has been reported as an efficient strategy for oncological outcomes and has been adopted by many liver centers[5,6]. A real novelty on 2 staged surgical procedures that has recently been proposed, is the advent of associating liver partition and PVL for staged hepatectomy (ALPPS)[7]. The technique was first performed by Schlitt et al[8] of Regensburg in 2007 and first presented to a German Congress in 2010. After that the technique spread all over the world[9]. This is rapidly gaining great interest from the surgical community leading to debate[10] and even proposals for a reinterpretation of methods[10-12], and giving a new hope to a large number of patients traditionally judged unresectable[13]. Despite an “explosion” of publications and case reports in the literature during the last 2 years, a lot of questions remain concerning safety and effectiveness of this method[14]. Given the large amount of surgical experience world wide[15], and the critical appraisals of some surgical groups, in the present article we would like to summarize the main surgical aspects, open questions and express our point of view on this method in the light of our preliminary experience[16] and other published data, in the era of two-stage treatment of CRLM[17].
The first aim of introducing this technique by Regensburg’s group was to enhance liver hypertrophy after portal ligation increasing the ischemia effect on future liver remnant (FLR)[16]. The concept on which this technique is based seems to be an old finding[18], but of course revisited and reinvented or recombined in the light of new problems of CRLM surgery[19]. The assumption is that any closure of the right portal branch is followed by a reactive perfusion of “deportalized” liver, from controlateral one, through the intrahepatic branches and collaterals presents between the 2 lobes[20]. This aspect was recently confirmed by a clinical study[21].
The technique consists in an association of classical right portal branch ligation together with liver parenchyma surgical split. The split could be conducted following the falciform ligament line (splitting segment 2-3 from the rest of the liver) as originally described[7], or even atypically adding 4b segment as previously shown in our video[22]. To split the liver avoiding manipulation and obtaining a better bleeding control, such as a clear anatomic line of parenchymal transection, we usually perform an anterior approach[23]. Between the right and left split hemilivers a plastic sheet or bag should be positioned in order to avoid cicatrisation with the disappearance of the resection line. In addition some atypical resection of additional metastases (1 or 2) in the future liver remnant must be performed. We have extended the indication to ALPPS also for bilateral CRLM with little FLR (< 30%) or even < 40% with damaged liver parenchyma; and during the first step of the procedure we resect metastases on FLR. Other groups used more restricted indications reserving ALPPS only for patients without metastases on FLR[7].
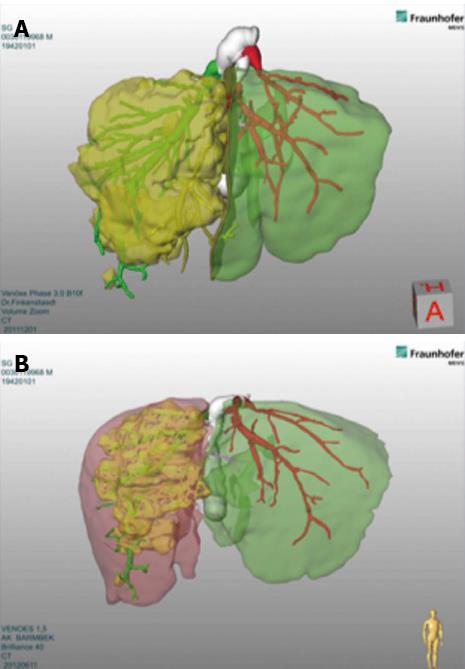
In the original description the Authors waited for about 8-10 d before performing the 2nd step of specimen removal[7]. Other Authors usually wait 7 d[24], while our group usually performs the 2nd step after 12 d. A computed tomography (CT) scan after 1st step, in 7-10 d, is mandatory in order to evaluate volumetric gain. We routinely use 3D-reconstruction and Volumetry performed by MEVIS® system using Hepavision® software[22] (Figure 1). The key role of CT-volumetry with 3D-reconstructions for this novel method was later confirmed by another study[25]. This technique showed that the speed of hypertrophy and also the percentage of volume gain were more enhanced than with classical methods such as PVE or PVL[7].
Some technical variants were quickly introduced by some surgeons. Some authors, in fact, proposed the laparoscopic approach[11,26], citing some advantages of laparoscopy in avoiding adhesions thus making the 2nd step easier[27]. Even if ALPPS was developed for extended right hepatectomy, 3 main strategies were recently standardized to use ALPPS not only for right hepatectomies but even for extended left hepatectomies[28].
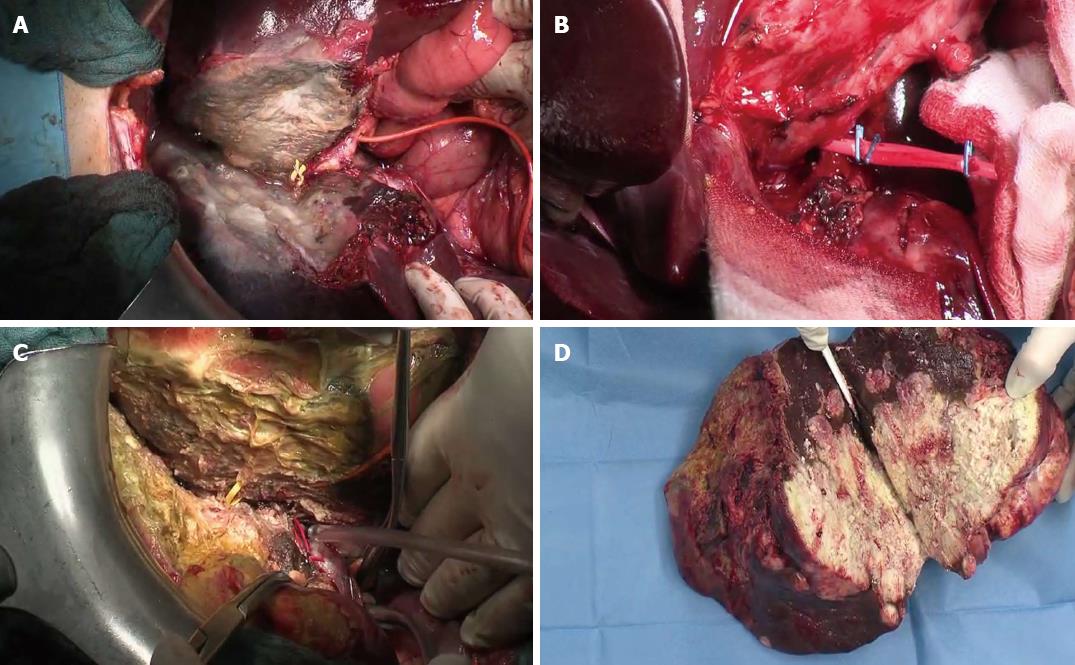
We proposed the following technical modifications in addition to referred routine use of 3D-reconstruction in order to improve the safety of the procedure: (1) Do not use the plastic bag or sheet but interpose only a fibrillar mesh (Figure 2A); (2) Use of a colored plastic loop to leave in situ for the 2nd step (yellow for right biliary duct and red for hepatic artery) in order to find it very easily and quickly during the challenging 2nd step (Figure 2B and C); and (3) Routine use of T-drain in order to reduce the risk of biliary leak and biomass (main referred surgical complication after preliminary reports (Figure 2B and C). Additionally we enhanced the significance of complete liver mobilization (apparently in contradiction with the anterior approach!) as previously described as a preliminary maneuver of surgical step 1, that in this technique, in our opinion, is of value not only for a complete manual and ultrasonography exploration of both lobes, but also to enhance the ischemia effect, avoid collateralisation through ligaments’ vessels and increase the hypertrophy effect of ALPPS. On the other hand some surgeons criticized[10,15] the technique, questioning: complexity of procedure, high risk of 2 very close big operations, additional morbidity and reported mortality, and uncertainty of long-term oncological results. If detractors have made some logical considerations about the proposed method, also enthusiasts of ALPPS have been engaged in reporting positive results[11,16,17]. It should be underlined that some of the most talented surgeons use ALPPS[9], testifying to the potential of this technique, trying to find the best indications (Table 1).
| Author | Cases (n) | Hypertrophy (range) | Days of interval (range) | Morbidity | Mortality |
| Schnitzbauer et al[7] | 25 | 74% (21-192) | 8 (4-8) | 64% | 12% |
| Conrad et al[11] | 1 | 44.80% | 9 | 0% | 0% |
| Robles Campos et al[12] | 1 | 57% | 7 | 0% | 0% |
| Dokmak et al[15] | 8 | 70% (5-147) | 7 | 87.50% | 25% |
| Donati et al[16] | 8 | 80% (66-200) | 10 (7-21) | - | - |
| Knoefel et al[17] | 7 | 63% | 6 (4-8) | 57.20% | 14.20% |
| Machado et al[26] | 8 | 88%1 | 9 | 0% | 0% |
| Machado et al[27] | 1 | 159% | 21 | 0% | 0% |
| Hahn et al[37] | 1 | 94% | 9 | 0 | 0 |
| Sala et al[49] | 10 | 82% (31-140) | 7 | 40% | 0% |
| Andriani[50] | 2 | - | 30 | 0% | 0% |
| Torres et al[51] | 1 | - | - | - | |
| Li et al[52] | 9 | 87.20% | 13 | 22.20% | 22.20% |
We have also expressed some considerations related to our preliminary data in a previous article[16], however, the ideas and criticisms raised during this last year convinced us to introduce, in this debate, some other considerations.
First of all it should be taken into consideration that ALPPS is mainly indicated for patients that have to undergo a right trisegmentectomy. This extended liver resection is known to be at particular risk of postoperative liver failure[4], the combination in a 2 staged procedure is forced in these patients by a judgment of not resectability with other established 2 stage surgical strategies[29-31]. Therefore this cohort of patients is “per se” a group of very sick patients with advanced disease, traditionally not resectable CRLM and therefore destined for palliative treatment. The novelty of this method is the percentage gain of patients to resectability. Thus we are convinced that the questionable additional risk related to the technique could be acceptable in the light of resectability gain. Doubtless, also other one stage combined proposed strategies could be taken into account in selected groups of patients[32]. However, very aggressive chemotherapic regimens, in many cases, are nowadays forcing surgeons to find new technical solutions, sometimes delaying radical treatment in order to achieve patients’ safety. We also think that the potential of ALPPS was wrongly judged by some eminent colleagues only because it was tested on very challenging indications (duodenocephalopancreatectomy and extended hemihepatectomy for biliary tract tumors)[15] leading to high morbidity and mortality rates. We espressed our opinion that the main indication for ALPPS seems to be for CRLM in selected patients, as stated in other papers[16]. In patients affected by bilateral CRLM, the proposed method regained resectability also in apparently not resectable patients, increasing safety of resection and lowering risk of postoperative liver failure, enhancing, in a very short time (average about 7 d), the great hypertrophy potential of liver parenchyma. Undoubtedly ALPPS, compared to traditional PVE or PVL, allows a tremendously quick FLR growth (22% vs 3% growth the day after the procedure)[16]. Of course our previous considerations should be considered in the light of no published long-term oncologic results. In consideration of the right moment to resect after split, this aspect should be carefully taken into account in patients submitted to several cycles of neoadjuvant chemotherapy. As we demonstrated in a previous publication, a long wait (about 4 wk) after split can allow a volumetric gain of 200%, but with the disadvantage of a more difficult second surgical step[23]. It is foreseeable that with the rapid diffusion of new neoadjuvant chemotherapic regimens (chemo first approach), the need for ALPPS, as a safe alternative strategy to the classic 2 stage approach, will increase; in fact, more patients are gaining, and will gain, resectability due to partial or sometimes full response to new chemotherapic protocols[33,34]. Thus a multidisciplinary approach of CRLM[35], starting with aggressive neoadjuvant regimen, under indications of institutional tumor boards, will push more patients to ALPPS. On the other hand, ALPPS makes the resection safer after neoadjuvant chemotherapy, reducing the risk of postoperative liver failure.
Even with the big enthusiasm for this technique and surge in the number of centers adopting it over the last 2 years, even in a episodical manner, leading to a lot of case reports[36-38], some considerations must be made. First of all we should consider that the method has not yet been tested in an evidence based manner, only preliminary experience is available, publishing dishomogeneous data. Even the technique has not yet been standardized. The big question is if oncologic long-term results are acceptable, if a gain, in terms of quality of life and time gained, could balance the big risk of complications and mortality. Whether the stimulation of liver hypertrophy could also accelerate tumor progression is also an open question still debated since the time of classic PVE, PVL techniques[39]. Recently, Van Gulik’s group has shown how tumor progression could clearly be stimulated by PVE only[40], reporting the objections about the short time-frame of ALPPS among speculations, because the same phenomenon is observed in PVE and PVL[41]. Even a study by Pamecha et al[42] first experimentally and then confirmed by Maggiori et al[43], showed a clear tumor progression after PVE so that about one third of patients cannot undergo the second step after embolization because of tumor progression. Obviously one could speculate that if ALPPS allows a bigger and quicker liver regeneration, the same stimulation could realize an intensive and quicker tumor progression. An early recurrence was occasionally reported among disomogeneous published experiences[7], but more data are expected in the next years and of course also results of international register.
If CRLM is to be considered the best indication in other tumours (for example Neuroendocrine tumor metastases) with slower biology and tumor progression, patients could benefit from such a method, this remains one of the main questions. Thus the great advantage of volumetric gain must be taken with the above mentioned open questions before establishing the method in clinical practice. The method remains very challenging and not only for liver surgeons, but for extremely skilled liver centers and must be approached in a multidisciplinary manner. More effort must be made to reduce the morbility and mortality associated with ALPPS.
Of course it must be taken into consideration that some other established methods of obtaining FLR hypertrophy are less risky and whenever possible should be the first choice in planning staged surgical strategies[44]. Nevertheless, also if PVE is, to date, the most used technique and considered the standard procedure to enhance FLR, about 30% of patients never undergo complete resection because of insufficient hypertrophy or tumor progression on FLR. Furthermore, as in a recent systematic review the two stage hepatectomy with traditional strategies has shown an certain morbidity, that appears comparable with the morbidity of ALPPS (17% after first step and 40% after the second one)[6]. Therefore, in well selected cases, in limit-cases, or when PVE failed to gain volumetric enhancement of FLR[17,45], sometimes ALPPS seems to be the only reasonable-feasible option to achieve resectability. The additional morbidity and mortality referred in the bigger reports respectively up to 44% and 12% could be accepted as additional risks only in the light of “no other choices”, even if we need more scientific studies to confirm this. Furthermore, it should be taken into account that reported high mortality rates are referred to very initial experiences in very small groups of patients, in which also 1 death strongly influences overall mortality rates. However, also 3rd referral hepatobiliary centers need a learning curve to optimize the procedure. Some proposed technical details could reduce the “surgical risk” also shortening the time of the second procedure[15]. Due to ethical limits to clinical experimentation and the difficulty in recruiting a reasonable group of highly selected patients, an online world register was created (see international register: http://www.alpps.net). Some detractors of ALPPS have recently published a study comparing traditional PVE efficacy and safety compared with published data on ALPPS, concluding to be in favour of traditional and well established strategies[46]. However, despite confirming that PVE and similar techniques are still the standard of care, the referred study suffers from some BIAS in comparison, therefore conclusions are not well addressed by the study and a definitive conclusion cannot be made. The challenge of the ongoing study will be, despite the BIAS of patient collection from many different centers, to establish some kind of evidence of safety (as declared by many authors and criticized by others), usefulness (long-term oncologic results), best indications, and in our opinion also guidelines to standardize the preoperative flow-chart, surgical timing and steps. It is foreseeable that ALPPS could gain a position also among feasible surgical strategies for the complex scenario of Klatskin tumours[47,48] in order to extend the resectability rate as we stated in a previous article[16], and as confirmed by recently reported experiences[35,49]. Despite all the potential of this technique, to date the scientific evidence should be still considered as a phase 1 clinical trial; we believe that the method, in consideration of all the above mentioned open questions at the moment, should be adopted only by extremely well-trained and experienced Hepatobiliary Surgical Centres.
The problems on the table are many and the technique needs to be defined, maybe first on acceptable indications and long-term results, in order to achieve the current position of ALPPS not only in the surgical management of CRLM, but also in its greater potential to treat other liver tumours. Therefore, in conclusion, we think that the ALPPS proposal should be considered the “real novelty” in the CRLM surgical landscape of the last 3 years and despite the enthusiastic view to “change the face of liver surgery” as suggested by other Authors[10], we prefer to say that it is foreseeable that such a method will gain, after the physiological period of experimentation and publication of the first large clinical studies, an important position among the surgical strategy options for the surgeon managing bilateral colorectal liver metastases, even maybe for a restricted and well-selected subgroup of patients.
P- Reviewers Cucchetti A, Homayounfar K S- Editor Zhai HH L- Editor A E- Editor Ma S
| 1. | Adams RB, Aloia TA, Loyer E, Pawlik TM, Taouli B, Vauthey JN. Selection for hepatic resection of colorectal liver metastases: expert consensus statement. HPB (Oxford). 2013;15:91-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 217] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 2. | Steele G, Ravikumar TS. Resection of hepatic metastases from colorectal cancer. Biologic perspective. Ann Surg. 1989;210:127-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 407] [Cited by in F6Publishing: 396] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 3. | Okabe H, Beppu T, Nakagawa S, Yoshida M, Hayashi H, Masuda T, Imai K, Mima K, Kuroki H, Nitta H. Percentage of future liver remnant volume before portal vein embolization influences the degree of liver regeneration after hepatectomy. J Gastrointest Surg. 2013;17:1447-1451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713-724. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1224] [Cited by in F6Publishing: 1591] [Article Influence: 122.4] [Reference Citation Analysis (0)] |
| 5. | Brouquet A, Abdalla EK, Kopetz S, Garrett CR, Overman MJ, Eng C, Andreou A, Loyer EM, Madoff DC, Curley SA. High survival rate after two-stage resection of advanced colorectal liver metastases: response-based selection and complete resection define outcome. J Clin Oncol. 2011;29:1083-1090. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 293] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 6. | Lam VW, Laurence JM, Johnston E, Hollands MJ, Pleass HC, Richardson AJ. A systematic review of two-stage hepatectomy in patients with initially unresectable colorectal liver metastases. HPB (Oxford). 2013;15:483-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 7. | Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, Fichtner-Feigl S, Lorf T, Goralcyk A, Hörbelt R. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 837] [Cited by in F6Publishing: 930] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 8. | Schnitzbauer A, Lang S A, Fichtner-Feigl S, Loss M, Kroemer A, Goessmann H, Farkas SA, Kirchner G, Jung EM, Scherer MN. In situ split with portal vein ligation induces rapid left lateral lobe hypertrophy enabling two-staged extended right hepatic resection. Berlin: Oral Presentation 2010; 35. [Cited in This Article: ] |
| 9. | Clavien PA, Lillemoe KD. Note from the editors on the ALPPS e-Letters-to-the-Editor. Ann Surg. 2012;256:552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Aloia TA, Vauthey JN. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): what is gained and what is lost? Ann Surg. 2012;256:e9; author reply e16-e19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 11. | Conrad C, Shivathirthan N, Camerlo A, Strauss C, Gayet B. Laparoscopic portal vein ligation with in situ liver split for failed portal vein embolization. Ann Surg. 2012;256:e14-e5; author reply e14-e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Robles Campos R, Paricio PP, Conesa AL, Hernández CM, Pérez RG, Quiñonero MF. [A new surgical strategy for multiple multiple bilobular liver metastases: right portal occlusion and torniquet in the parenchyma section line]. Cir Esp. 2012;90:191-196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Loos M, Friess H. Is there new hope for patients with marginally resectable liver malignancies. World J Gastrointest Surg. 2012;4:163-165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Kokudo N, Shindoh J. How can we safely climb the ALPPS? Updates Surg. 2013;65:175-177. [PubMed] [Cited in This Article: ] |
| 15. | Dokmak S, Belghiti J. Which limits to the “ALPPS” approach? Ann Surg. 2012;256:e6; author reply e16-e17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Donati M, Stavrou GA, Basile F, Gruttadauria S, Niehaus KJ, Oldhafer KJ. Combination of in situ split and portal ligation: lights and shadows of a new surgical procedure. Ann Surg. 2012;256:e11-e2; author reply e11-e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Knoefel WT, Gabor I, Rehders A, Alexander A, Krausch M, Schulte am Esch J, Fürst G, Topp SA. In situ liver transection with portal vein ligation for rapid growth of the future liver remnant in two-stage liver resection. Br J Surg. 2013;100:388-394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 18. | Sotiropoulos GC, Kouraklis G. The ALPPS procedure for extended indications in liver surgery: an old finding applied in surgical oncology. Ann Surg. 2013;257:e26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Schnitzbauer AA, Lang SA, Lang H, Schlitt HJ. Reply to letter: “The ALPPS procedure for extended indications in liver surgery: an old finding applied in surgical oncology”. Ann Surg. 2013;257:e27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Wilms C, Mueller L, Lenk C, Wittkugel O, Helmke K, Krupski-Berdien G, Rogiers X, Broering DC. Comparative study of portal vein embolization versus portal vein ligation for induction of hypertrophy of the future liver remnant using a mini-pig model. Ann Surg. 2008;247:825-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | van Lienden KP, Hoekstra LT, Bennink RJ, van Gulik TM. Intrahepatic Left to Right Portoportal Venous Collateral Vascular Formation in Patients Undergoing Right Portal Vein Ligation. Cardiovasc Intervent Radiol. 2013;Mar 13; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Oldhafer KJ, Donati M, Maghsoudi T, Ojdanić D, Stavrou GA. Integration of 3D volumetry, portal vein transection and in situ split procedure: a new surgical strategy for inoperable liver metastasis. J Gastrointest Surg. 2012;16:415-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Oldhafer KJ, Donati M, Lipp M, Keller B, Ojdanic D, Stavrou GA. [Anterior approach liver resection with the liver hanging maneuver. Technique and indications]. Chirurg. 2012;83:65-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Alvarez FA, Ardiles V, Sanchez Claria R, Pekolj J, de Santibañes E. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): tips and tricks. J Gastrointest Surg. 2013;17:814-821. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 25. | Ulla M, Ardiles V, Levy-Yeyati E, Alvarez FA, Spina JC, Garcia-Mónaco RD, De Santibañes E. New surgical strategy to induce liver hypertrophy: role of MDCT-volumetry to monitor and predict liver growth. Hepatogastroenterology. 2013;60:337-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 8] [Reference Citation Analysis (0)] |
| 26. | Machado MA, Makdissi FF, Surjan RC. Totally laparoscopic ALPPS is feasible and may be worthwhile. Ann Surg. 2012;256:e13; author reply e16-e19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 27. | Machado MA, Makdissi FF, Surjan RC. ALPPS procedure with the use of pneumoperitoneum. Ann Surg Oncol. 2013;20:1491-1493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Gauzolino R, Castagnet M, Blanleuil ML, Richer JP. The ALPPS technique for bilateral colorectal metastases: three “variations on a theme”. Updates Surg. 2013;65:141-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Capussotti L, Muratore A, Baracchi F, Lelong B, Ferrero A, Regge D, Delpero JR. Portal vein ligation as an efficient method of increasing the future liver remnant volume in the surgical treatment of colorectal metastases. Arch Surg. 2008;143:978-982; discussion 982. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 30. | Broering DC, Hillert C, Krupski G, Fischer L, Mueller L, Achilles EG, Schulte am Esch J, Rogiers X. Portal vein embolization vs. portal vein ligation for induction of hypertrophy of the future liver remnant. J Gastrointest Surg. 2002;6:905-913; discussion 913. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 142] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 31. | Stavrou GA, Donati M, Ringe KI, Peitgen HO, Oldhafer KJ. Liver remnant hypertrophy induction--how often do we really use it in the time of computer assisted surgery? Adv Med Sci. 2012;57:251-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Adam R, Laurent A, Azoulay D, Castaing D, Bismuth H. Two-stage hepatectomy: A planned strategy to treat irresectable liver tumors. Ann Surg. 2000;232:777-785. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 587] [Cited by in F6Publishing: 522] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 33. | Ye LC, Liu TS, Ren L, Wei Y, Zhu DX, Zai SY, Ye QH, Yu Y, Xu B, Qin XY. Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastases. J Clin Oncol. 2013;31:1931-1938. [PubMed] [Cited in This Article: ] |
| 34. | Loupakis F, Schirripa M, Caparello C, Funel N, Pollina L, Vasile E, Cremolini C, Salvatore L, Morvillo M, Antoniotti C. Histopathologic evaluation of liver metastases from colorectal cancer in patients treated with FOLFOXIRI plus bevacizumab. Br J Cancer. 2013;108:2549-2556. [PubMed] [Cited in This Article: ] |
| 35. | Donati M, Basile F. New trends in the multidisciplinary treatment of liver tumors. Future Oncol. 2013;9:1093-1096. [PubMed] [Cited in This Article: ] |
| 36. | Skipenko OG, Bedzhanian AL, Bagmet NN, Shatverian GA, Polishchuk LO, Chardarov NK. [Novel approach to two-stage hepatic surgery (in situ splitting)]. Khirurgiia (Mosk). 2013;37-41. [PubMed] [Cited in This Article: ] |
| 37. | Hahn O, Dudás I, Pajor P, Györke T, Korom C, Zsirka-Klein A, Kupcsulik P, Harsányi L. [ALPPS (Associated Liver Partition and Portal vein ligation for Staged hepatectomy) -- faster and greater growth of liver]. Magy Seb. 2013;66:21-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Govil S. Rapid improvement in liver volume induced by portal vein ligation and staged hepatectomy: the ALPPS procedure. HPB (Oxford). 2012;14:874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Lim C, Cauchy F, Azoulay D, Farges O, Ronot M, Pocard M. Tumour progression and liver regeneration--insights from animal models. Nat Rev Gastroenterol Hepatol. 2013;10:452-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Hoekstra LT, van Lienden KP, Doets A, Busch OR, Gouma DJ, van Gulik TM. Tumor progression after preoperative portal vein embolization. Ann Surg. 2012;256:812-817; discussion 817-818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 41. | Hayashi S, Baba Y, Ueno K, Nakajo M, Kubo F, Ueno S, Aikou T, Komokata T, Nakamura N, Sakata R. Acceleration of primary liver tumor growth rate in embolized hepatic lobe after portal vein embolization. Acta Radiol. 2007;48:721-727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 42. | Pamecha V, Levene A, Grillo F, Woodward N, Dhillon A, Davidson BR. Effect of portal vein embolisation on the growth rate of colorectal liver metastases. Br J Cancer. 2009;100:617-622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 43. | Maggiori L, Bretagnol F, Sibert A, Paradis V, Vilgrain V, Panis Y. Selective portal vein ligation and embolization induce different tumoral responses in the rat liver. Surgery. 2011;149:496-503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 44. | Schadde E, Slankamenac K, Breitenstein S, Lesurtel M, De Oliveira M, Beck-Schimmer B, Dutkowski P, Clavien PA. Are two-stage hepatectomies associated with more complications than one-stage procedures? HPB (Oxford). 2013;15:411-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 45. | Torres OJ, Fernandes Ede S, Oliveira CV, Lima CX, Waechter FL, Moraes-Junior JM, Linhares MM, Pinto RD, Herman P, Machado MA. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): the Brazilian experience. Arq Bras Cir Dig. 2013;26:40-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 46. | Shindoh J, Vauthey JN, Zimmitti G, Curley SA, Huang SY, Mahvash A, Gupta S, Wallace MJ, Aloia TA. Analysis of the efficacy of portal vein embolization for patients with extensive liver malignancy and very low future liver remnant volume, including a comparison with the associating liver partition with portal vein ligation for staged hepatectomy approach. J Am Coll Surg. 2013;217:126-133; discussion 133-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 168] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 47. | Donati M, Stavrou GA, Oldhafer KJ. Laparoscopic resections for hilar cholangiocarcinomas: a critical appraisal. Dig Surg. 2011;28:277-278; author reply 279-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 48. | Donati M, Basile F. Resectability criteria for Klatskin tumours: The “Black Run” of liver surgery. JST. 2012;2:1-3. [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 49. | Sala S, Ardiles V, Ulla M, Alvarez F, Pekolj J, de Santibañes E. Our initial experience with ALPPS technique: encouraging results. Updates Surg. 2012;64:167-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 50. | Andriani OC. Long-term results with associating liver partition and portal vein ligation for staged hepatectomy (ALPPS). Ann Surg. 2012;256:e5; author reply e16-e19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 51. | Torres OJ, Moraes-Junior JM, Lima e Lima NC, Moraes AM. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): a new approach in liver resections. Arq Bras Cir Dig. 2012;25:290-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 52. | Li J, Girotti P, Königsrainer I, Ladurner R, Königsrainer A, Nadalin S. ALPPS in right trisectionectomy: a safe procedure to avoid postoperative liver failure? J Gastrointest Surg. 2013;17:956-961. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |