Published online Sep 28, 2013. doi: 10.3748/wjg.v19.i36.6035
Revised: June 3, 2013
Accepted: June 18, 2013
Published online: September 28, 2013
Processing time: 262 Days and 5.8 Hours
AIM: To evaluate weight loss and surgical outcomes of Roux-en-Y gastric bypass (RYGB) and laparoscopic adjustable gastric band (LAGB).
METHODS: Data relating to changes in body mass index (BMI) and procedural complications after RYGB (1995-2009; n = 609; 116M: 493F; 42.4 ± 0.4 years) or LAGB (2004-2009; n = 686; 131M: 555F; 37.2 ± 0.4 years) were extracted from prospective databases.
RESULTS: Pre-operative BMI was higher in RYGB than LAGB patients (46.8 ± 7.1 kg/m2vs 40.4 ± 4.2 kg/m2, P < 001); more patients with BMI < 35 kg/m2 underwent LAGB than RYGB (17.1% vs 4.1%, P < 0.0001). BMI decrease was greater after RYGB. There were direct relationships between weight loss and pre-operative BMI (P < 0.001). Although there was no difference in weight loss between genders during the first 3-year post-surgery, male LAGB patients had greater BMI reduction than females (-8.2 ± 4.3 kg/m2vs -3.9 ± 1.9 kg/m2, P = 0.02). Peri-operative complications occurred more frequently following RYGB than LAGB (8.0% vs 0.5%, P < 0.001); majority related to wound infection. LAGB had more long-term complications requiring corrective procedures than RYGB (8.9% vs 2.1%, P < 0.001). Conversion to RYGB resulted in greater BMI reduction (-9.5 ± 3.8 kg/m2) compared to removal and replacement of the band (-6.0 ± 3.0 kg/m2). Twelve months post-surgery, fasting glucose, total cholesterol and low density lipoprotein levels were significantly lower with the magnitude of reduction greater in RYGB patients.
CONCLUSION: RYGB produces substantially greater weight loss than LAGB. Whilst peri-operative complications are greater after RYGB, long-term complication rate is higher following LAGB.
Core tip: Roux-en-Y gastric bypass (RYGB) produces substantially greater weight loss and resolution of co-morbidities than laparoscopic adjustable gastric band (LAGB) in a community setting, in both the short- and long-term. Although peri-operative complications are higher with RYGB than LAGB, which are non-fatal and mostly related to wound infection, the long-term complication rate is higher after LAGB. Where LAGB fails to induce or maintain weight loss, RYGB appears to be the superior salvage procedure. The better outcomes for LAGB in males compared to females after 3 years post-surgery are intriguing and needs further confirmation.
- Citation: Nguyen NQ, Game P, Bessell J, Debreceni TL, Neo M, Burgstad CM, Taylor P, Wittert GA. Outcomes of Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding. World J Gastroenterol 2013; 19(36): 6035-6043
- URL: https://www.wjgnet.com/1007-9327/full/v19/i36/6035.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i36.6035
Obesity is increasing in prevalence in the Western world and affects approximately 30% of the population[1,2]. It is associated with significant co-morbidities including diabetes, cardiovascular disease and obstructive sleep apnoea[1,3]. Unfortunately, medical treatment with lifestyle modification or pharmacotherapy is only modestly effective with a high relapse rate[4,5]. Bariatric surgery is recommended for patients with a body mass index (BMI) > 40 kg/m2 or BMI > 35 kg/m2 with significant co-morbidities[2,3]. Two commonly performed bariatric procedures are Roux-en-Y gastric bypass (RYGB) and laparoscopic gastric banding (LAGB)[2,3].
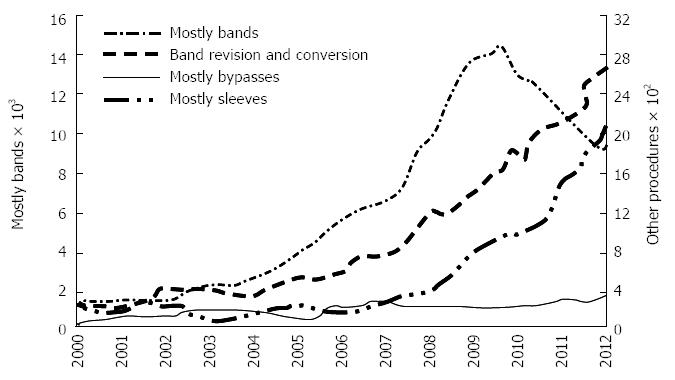
Currently, the choice between these bariatric procedures is based mainly on patient and surgeon preference, and varies significantly between regions of the world. In contrast to the United States and Europe, LAGB is the most common procedure in Australia (90%; with -10% RYGB)[6,7], perhaps because LAGB is perceived as a safer, minimally invasive, fully reversible and adjustable procedure[6,7]. In Australia, the number of LAGB procedures increased by 10 times over the last decade, as compared to the stable rate of RYGB procedures (Figure 1). This is despite the majority of available data, including the recent systematic and network meta-analysis of randomised trials on weight loss outcome at 1 year, indicating that RYGB produces greater weight loss with more frequent resolution of type 2 diabetes, hypertension, dyslipidemia and sleep apnoea (OSA)[3,8-10].
For LAGB, meticulous follow up has been suggested to play an important role in achieving and maintaining the weight loss, and this may be responsible for some of the impressive weight loss reported by Australian LAGB centers[7,11-13]. Alternatively drop-outs or “treatment failures” may be under-reported. Thus, the aim of the current study was to compare weight loss and surgical outcomes of RYGB and LAGB from two large, prospectively maintained surgical databases.
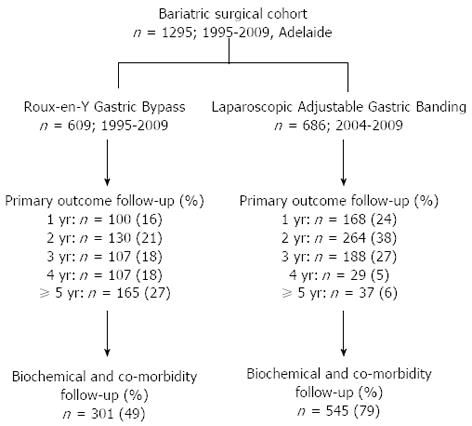
Data from two clinical databases of obese patients who underwent primary RYGB or LAGB were reviewed. The data have been collected prospectively and maintained by two experienced bariatric surgeons performing predominantly either RYGB (PG) or LAGB (JB) in Adelaide, South Australia. The RYGB cohort included all patients who had surgery from 1995 to 2009, by either open or laparoscopic techniques. The LAGB group included all patients who underwent the procedure from 2004 to 2009 (Figure 2). The RYGB cohort included both public and private patients, whereas all patients in the LAGB group were treated in private hospitals.
For both RYGB and LAGB cohorts, the patients were assessed individually by the respective surgeon and a dietitian as well as, in most cases, a multi-disciplinary team (physician, exercise physiologist, psychologist) prior to surgery. In general, the decision to undertake bariatric surgery followed the recommendations of the American Society for Metabolic and Bariatric Surgery Clinical Issues Committee[1].
Both databases were kept electronically (Microsoft Office Access® for RYGB cohort and Filemaker Pro V8 for LAGB cohort) and maintained by the surgeon and the practices. For all patients, information on demographics, pre-operative and post-operative body mass index, and pre-operative co-morbidities were recorded. Details on the peri-operative and long-term complications were also recorded for the affected cases. Scanned copies of serial laboratory measurements (fasting glucose, lipid profile and liver function test) were available in approximately 50% of cases.
Data on patient demographics, date and type of procedure and changes in BMI over the period of follow up were exported to excel files from Microsoft Office Access®. Data regarding obesity related co-morbidities, medications, resolution of co-morbidities, and serial laboratory measurements were collected by reviewing each patient’s files (both paper and electronic). Complete serial data on blood glucose, lipid profiles and changes on co-morbidities over the first 12 mo after surgery were available for 301 RYGB and 545 LAGB patients.
Open RYGB procedure: After midline incision, a gastric pouch of approximately 25 mL was created by stapling off the proximal stomach with a TA 90B four-row stapler (Autosuture®, Covidien®), or the stomach was divided using a linear stapler (GIATM, Covidien® or TLCTM, Ethicon®) stapler. The pouch volume was not measured using upper gastrointestinal endoscopy or a balloon device. A retrocolic and retrogastric 100-cm Roux limb was anastomosed to the gastric pouch using a two-layered hand-sewn technique, with an outer 3/0 polypropylene non-absorbable continuous layer and an inner 3/0 PDS absorbable continuous suture. A hand-sewn side-to-side jejuno-jejunal anastomosis was performed. The integrity of the gastro-jejunostomy was tested with methylene blue.
Laparoscopic RYGB procedure: A five-port technique was used. The stomach was transected with a laparoscopic linear stapler (Endo-GIATM, Covidien® or EndopathTM, Ethicon®). The two anastomoses were either performed with a hand-sewn technique or with stapling devices (EEATM OrVilTM XL circular stapler, Endo-GIATM, Covidien®). Again, the integrity of the gastro-jejunostomy was tested with methylene blue.
Laparoscopic adjustable gastric banding procedure: Commercially available laparoscopic adjustable gastric bands from Allergan Inc (Lap Band; Allergan, Inc, Irvine, California) were used. The size of the gastric band system used was at the discretion of the surgeon. The band was inserted using the pars flaccida technique, which involved adequate exposure of the angle of His by retracting the gastric fundus inferiorly. Dissection was continued until the left crus of the diaphragm was completely exposed. A small incision in the avascular aspect of the gastro-hepatic ligament was created, and care was taken to identify and preserve the hepatic branch of the vagus nerve. Blunt dissection was used to create a space between the base of the right crus and its overlying peritoneum. A long grasper was then gently passed above the right crus, underneath the gastroesophageal junction, toward the angle of His. The lap-band was passed around the gastroesophageal junction and snapped in place and secured with 3 gastrogastric sutures. The band reservoir was filled with 3 mL of normal saline for APS bands, and 4 mL of normal saline for APL bands.
Patients from either centre followed a strict post-operative protocol. For both procedures, clear liquids were provided on the first post-operative day and a full-liquid diet on the second.
Patients undergoing RYGB were seen in the clinic 2 wk post-operatively, every 3 mo for the first year, and then annually thereafter. After LAGB, patients were asked to continue seeing a dietician, psychologist, exercise physiologist and a general practitioner with an interest in obesity and bariatric surgery. Most patients returned every 3 mo for the first year, and 6-monthly thereafter for patients who progressed well. Some patients may have seen the bariatric GP more frequently depending on progress and problems experienced. Band adjustments were most commonly performed at week 2 and 6 postoperatively, then every 3 mo for the first year (total of 5 visits). Adjustments to the band were performed according to manufacturer’s guidelines.
For both surgeries, most patients were advised to take supplementary vitamins (including vitamin D) and calcium.
Primary outcome measures: (1) Weight loss. This was expressed as change in BMI from baseline over the study duration; and (2) Complications and need for re-operation. Acute (< 30 d) and long-term (≥ 30 d) complications included use of unexpected drug therapy or imaging, total parenteral nutrition, a bedside procedure, blood transfusion, a hospital stay longer than twice the median stay, diagnostic or therapeutic endoscopy, re-operation (with or without organ resection or anastomotic revision), or death.
Secondary outcome measures: (1) Diabetes mellitus and hyperlipidemia. As data regarding the need for anti-diabetic or anti-cholesterol medications were incomplete, only improvement rather than “resolution” of diabetes mellitus or hyperlipidemia could be assessed, and the change from baseline to 12 mo was compared between the procedures. Improvement in diabetes mellitus was defined as a fasting blood glucose < 5.5 mmol/L. Improvement in hyperlipidemia was expressed as a percentage of patients who had normalization of plasma total cholesterol (level < 5.5 mmol/L) or plasma triglycerides (level < 2.0 mmol/L); and (2) Obstructive sleep apnoea. Resolution of obstructive sleep apnea was defined as the absence of requirement for continuous positive airway pressure after surgery.
Data were expressed as mean ± SD, unless stated otherwise. Comparison of variables between the two surgical groups was undertaken using χ2 tests for categorical data and independent t-test for continuous data sets. The differences in changes in weight loss outcome over time between the groups were compared using Kaplan Meier analysis. All analyses were performed using GraphPad Prism statistical software, version 6 (GraphPad Software Inc., La Jolla, CA, United States).
A total of 1295 patients was included in the study; 609 underwent RYGB (116M:493F; age: 42.4 ± 10.5 years) and 686 underwent LAGB (131M:555F; age: 37.2 ± 9.4 years). For the RYGB cohort, 13% (78) of procedures were performed laparoscopically. The initial BMI was significantly higher in patients who underwent RYGB than LAGB (46.8 ± 7.1 kg/m2vs 40.4 ± 4.2 kg/m2, P < 0.01). Overall, 161/1295 (12%) patients underwent bariatric surgery with an initial BMI between 30 and 35 kg/m2, and it was more prevalent in patients who underwent LAGB (128/686 vs 33/609, P < 0.0001). The median number of follow up visits after surgery was 6 in both groups, with 63% of RYGB patients and 38% of LAGB patients had follow-up duration of greater than 3 years (Table 1 and Figure 2).
| RYGB (n = 609) | LAGB (n = 686) | |
| Gender (M:F) | 116: 493 | 131: 555 |
| Age (yr) | 42.4 ± 10.5b | 37.2 ± 9.4 |
| Initial BMI (kg/m2) | 46.8 ± 7.1b | 40.4 ± 4.2 |
| Co-morbidities (sub-group analysis) | (n = 301) | (n = 545) |
| Total co-morbidities | 216 (71.0) | 363 (66.6) |
| Type 2 diabetes mellitus (n) | 82 | 95 |
| Hypertension (n) | 51 | 157 |
| Hyperlipidemia (n) | 116 | 117 |
| Obstructive sleep apnoea (n) | 97b | 113 |
| Duration of follow-Median (yr) | 2.0 (1.0-5.0) | 1.6 (1.0-2.2) |
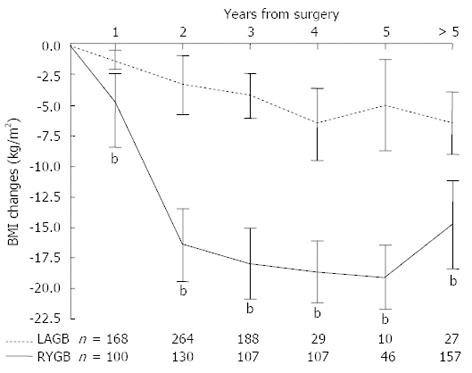
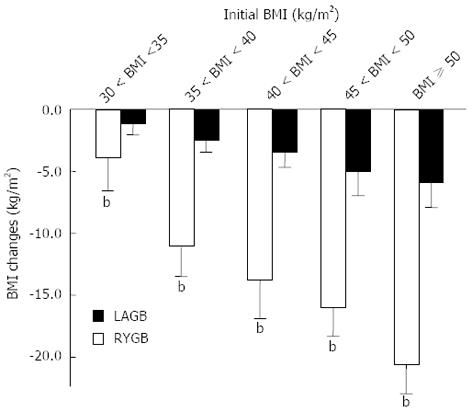
Overall, RYGB resulted in a significantly greater decrease in BMI than LAGB (-14.8 ± 3.4 kg/m2vs -2.9 ± 1.2 kg/m2, P < 0.0001). The greater reduction in BMI after RYGB over LAGB was observed at all time points, and the peak weight loss was observed at year 4 for both procedures (Figure 3). Irrespective of pre-operative BMI, weight reduction was greater after RYGB than LAGB (P < 0.001, Figure 4). For both surgical groups, there was a direct relationship between weight loss and the pre-operative BMI (P < 0.001) (Figure 4).
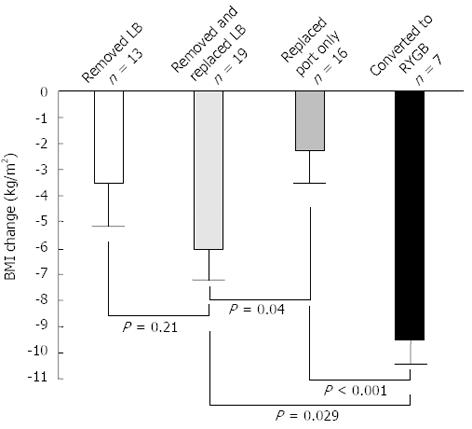
Peri-operative complications were higher with RYGB than LAGB (8.0% vs 0.5%, P < 0.001). The majority of the acute complications in the RYGB group were minor and did not require any surgical intervention (Table 2). Long-term complications necessitating corrective procedures were higher following LAGB than RYGB (8.9% vs 2.1%, P < 0.001). Conversion to RYGB resulted in a greater BMI reduction (-9.5 ± 3.8 kg/m2) as compared to removal and replacement of the band (-6.0 ± 3.0 kg/m2) (Figure 5).
| RYGB (n= 609) | LAGB (n= 686) |
| Acute complications (n) | |
| n = 49 (8.0%) | n = 3 (0.5%) (P < 0.0001 vs RYGB) |
| Wound infection (35) | Small leak, required band removal after 1 d (1) |
| Abdominal sepsis (3) | Post-op respiratory infection (1) |
| Splenic trauma (2) | Large wound haematoma, drained spontaneously (1) |
| DVT and PE (2) | |
| Endoscopy for stomal obstruction (4) | |
| Mechanical failure (3) | |
| Long-term complications (n) | |
| n = 13 (2.1%) | n = 61 (8.9%) (P < 0.0001 vs RYGB) |
| Reversal of RYGB (n = 1) | Removed and replaced LB (19) |
| Incisional hernia (n = 7) | Replacement of port (16) |
| Bowel obstruction (n = 5) | Removed LB (13) |
| Converted to RYGB (7) | |
| Stomal obstruction required dilation (3) | |
| Mechanical failure (3) | |
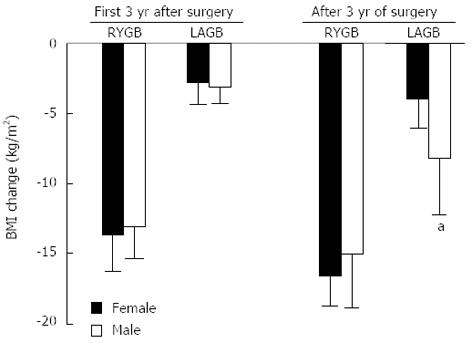
Pre-operative BMI was similar in males and females undergoing LAGB (41.1 ± 4.5 vs 40.3 ± 4.3 kg/m2), but greater in males than females undergoing RYGB (47.9 ± 4.9 vs 44.9 ± 3.8 kg/m2, P < 0.01). RYGB induced greater weight loss than LAGB in both genders (male: -13.8 ± 3.1 vs -3.5 ± 1.9 kg/m2; P < 0.0001; female: -15.0 ± 3.8 vs -2.9 ± 1.3 kg/m2, P < 0.0001), with no differences in weight loss between genders during the first 3 years of surgery. Thereafter LAGB males had a greater reduction in BMI than females (-8.2 ± 4.3 vs -3.9 ± 1.9 kg/m2, P = 0.02, Figure 6).
The rates of acute (male: 9/116 vs female: 40/493) or long-term complications (male: 2/116 vs female: 11/493) were similar in males and females after RYGB. In the LAGB cohort, however, longer term complications requiring corrective procedures, were less likely to occur in males than females (male: 2/131 vs female: 59/555, P < 0.001). The rate of acute complication for both genders was similar after LAGB (male: 0/131 vs female: 4/555).
There were no differences in age, gender, initial BMI and magnitude of weight loss between those patients for whom biochemical/co-morbidity data was, or was not, available (Table 3). Before surgery, there were no differences in the proportion of patients with diabetes mellitus, hyperlipidemia or hypertension (Table 1). The presence of significant co-morbidities (diabetes mellitus, hypertension, sleep apnoea) in patients who had initial BMI between 30-35 kg/m2 was 45% in both RYGB and LAGB groups.
| RYGB (n = 609) | LAGB (n = 686) | |||||
| Biochemical data (n = 301) | No biochemical data (n = 308) | P-value | Biochemical data (n = 545) | No biochemical data (n = 141) | P-value | |
| Gender (M:F) | 63:238 | 53:255 | > 0.05 | 101:444 | 30:111 | > 0.05 |
| Age (yr) | 42.9 ± 10.2 | 42.0 ± 10.6 | > 0.05 | 37.8 ± 9.4 | 37.1 ± 9.5 | > 0.05 |
| Initial BMI (kg/m2) | 45.9 ± 7.5 | 47.0 ± 7.4 | > 0.05 | 40.6 ± 4.1 | 40.3 ± 4.2 | > 0.05 |
| BMI reduction at 12 mo (kg/m2) | -6.5 ± 3.4 | -7.7 ± 4.6 | > 0.05 | -1.2 ± 0.8 | -1.4 ± 0.9 | > 0.05 |
| BMI reduction at 3 yr (kg/m2) | -18.2 ± 3.9 | -19.0 ± 4.9 | > 0.05 | -4.9 ± 2.6 | 4.3 ± 2.4 | > 0.05 |
Fasting blood glucose (33% vs 17%, P = 0.02), total cholesterol (54% vs 4%, P < 0.001), and plasma triglyceride (81% vs 27%, P < 0.0001) normalised more frequently after RYGB than after LAGB, respectively (Table 4). Fasting blood glucose, total cholesterol and triglyceride levels were significantly reduced 12 mo after both types of procedures, however the magnitude of improvement was greater and the onset of improvement was earlier after RYGB (Table 5). Both procedures resulted in a similar increase of plasma high-density lipoprotein.
| RYGB | LAGB | P-value | |
| (n = 301) | (n = 554) | (RYGB vs LAGB) | |
| Diabetes mellitus | |||
| Pre-operative | 83 (28) | 127 (23) | 0.34 |
| Normalization of fasting blood glucose1 | 26 (33) | 22 (17) | 0.02 |
| Hyper-cholesterolemia | |||
| Pre-operative | 125 (42) | 224 (41) | 0.77 |
| Normalization of total plasma cholesterol1 | 65 (52) | 9 (4) | < 0.001 |
| Hyper-triglyceridemia | |||
| Pre-operative | 63 (21) | 124 (22) | 0.86 |
| Normalization of plasma triglyceride1 | 51 (81) | 34 (27) | < 0.0001 |
| Obstructive sleep apnoea | |||
| Pre-operative | 100 (33) | 130 (23) | 0.02 |
| No longer required CPAP at 12 mo | 10 (10) | 4 (3) | 0.04 |
| RYGB | LAGB | P-value | |
| (n = 301) | (n = 554) | (RYGB vs LAGB) | |
| Fasting blood sugar | |||
| Prior to surgery | 5.9 ± 1.6 | 5.7 ± 1.7 | 0.12 |
| 6-month post-op | 5.3 ± 1.6b | 5.5 ± 1.9b | 0.57 |
| 12-month post-op | 5.0 ± 1.7b | 5.4 ± 1.6b | 0.02 |
| Total cholesterol | |||
| Prior to surgery | 5.2 ± 1.0 | 5.2 ± 1.0 | 0.65 |
| 6-month post-op | 4.2 ± 0.9b | 5.2 ± 1.0 | < 0.0001 |
| 12-month post-op | 4.3 ± 0.9b | 5.0 ± 1.0b | < 0.0001 |
| Total triglyceride | |||
| Prior to surgery | 1.7 ± 0.5 | 2.0 ± 0.6 | 0.44 |
| 6-month post-op | 1.2 ± 0.6b | 1.5 ± 0.6 | 0.01 |
| 12-month post-op | 1.1+0.4b | 1.5 ± 0.5 | 0.04 |
| HDL | |||
| Prior to surgery | 1.3 ± 0.3 | 1.3 ± 0.3 | 0.26 |
| 6-month post-op | 1.3 ± 0.3 | 1.4 ± 0.3b | 0.21 |
| 12-month post-op | 1.4 ± 0.3b | 1.5 ± 0.3b | 0.56 |
Patients who underwent RYGB were more likely to have obstructive sleep apnea (OSA: 32% vs 20%, P < 0.001; Table 3). The proportion of patients who had resolution of OSA were significantly higher after RYGB than after LAGB (10% vs 3%, P = 0.03; Table 4).
For patients who underwent bariatric surgery for morbid obesity this data shows that, while both procedures are safe, RYGB was associated with: (1) substantially greater weight loss; (2) lower fasting blood glucose, total cholesterol and triglyceride levels; (3) greater risk of acute non-fatal complications; and (4) lower rate of re-operation rate in long-term. Following band failure, conversion to RYGB resulted in a greater reduction in BMI, than band replacement, and may therefore be a preferable rescue procedure. This may be particularly relevant for patients with inadequate resolution of co-morbidities after LAGB. Furthermore, the current study demonstrates the differential impact of gender on weight loss and complications in patients who underwent LAGB but not RYGB. The greater weight loss observed in males 3 years post lap band insertion was associated with a significantly lower rate of long-term band related complications.
The findings of this study are consistent with the available data from both randomized and non-randomized clinical trials[3,8-10,14-18]. Currently, only 2 prospective randomized comparisons of RYGB against LAGB have been performed and both have methodological weaknesses[8,17]. Although the first study found better short-term weight loss and a lower number of weight loss failures after RYGB, the sample size was small (24 RYGB vs 27 LAGB) limiting the capacity to make definitive conclusions, particularly about uncommon adverse events[17]. The larger, second trial (111 RYGB vs 86 LAGB) confirmed the superior weight loss after RYGB as compared to LAGB, but was criticized for significant differences in baseline BMI between the groups and an unusual high rate of gastro-jejunostomy stricture[8]. Both studies showed a higher rate of peri-operative but lower incidence of later complications and need for re-operation after RYGB than LAGB[8,17]. Similar outcomes have been reported from pair-matched studies, as well as systematic and network meta-analyses[3,8-10,14-18].
The marked improvement or normalization of fasting blood glucose and lipid profile after both RYGB and LAGB in this current study are in keeping with previous studies[3,8-10,14-18]. The observation that, of the two procedures, RYGB has a much greater benefit for these co-morbidities, is consistent with previously reported data[3,9]. The greater proportion of patients with OSA in the RYGB group is most likely related to the greater initial BMI, which is a known risk factor for OSA. Similar, the greater resolution of OSA after RYGB, as compared to LAGB, is most likely related to the greater reduction in BMI, and the finding is consistent with the current literature[3].
The differences in acute and long-term complications in the current study are also in keeping with the current literature[3,8-10,14-18]. Given RYGB is a more complex operation with a longer operative time, it is not surprising that the prevalence of acute post-operative complications was higher after RYGB than LAGB. It is, however, important to note that none of the complications were fatal and most were related to wound infection and managed successful with medical therapy. Compared to the 1% mortality reported internationally, there were no surgical deaths amongst our RYGB cohort. The reasons for the absence of mortality may relate to the meticulous patient assessment and selection, pre-operative preparation, and anaesthetist with a special interest and expertise in dealing with complex bariatric patients. The impressively low rate of acute complications after LAGB in this report should be highlighted and is consistent with the current literature[3,8-10,14-18], suggesting that LAGB is an extremely safe bariatric procedure.
Except for the study of Nguyen et al[8], which was criticized for the unusually high rate of gastro-jejunostomy stricture after RYGB, our and other long-term follow-up studies have consistently shown a higher rate of long-term complication and the need for re-operation after LAGB as compared to RYGB[3,8-9].
The superior weight loss at 3 years after LAGB in males as compared with females may relate, at least in part, to a lower number of postoperative long-term complications. Other studies have either shown similar[14,19,20] or worse outcomes[8,21] for males than females after LAGB. One reason for this is the poor adherence of men to post-band advice and follow-up[22,23]. On the other hand, men are less likely to have over-eating disorders, are more willing to exercise if feeling well[22,23], and generally have better outcomes in most studies of diet induced weight loss[24]. In the most recent large study of LAGB procedures (n = 3000), the occurrence of proximal gastric pouch dilatation, a known risk factor for poor weight loss, was significantly more common in women than men (5.1% vs 1.3%), and was highest in younger women[23]. The possibility of selection biases by selectively chosen highly motivated males cannot be excluded. Regardless, better outcomes for LAGB in males than females indicates that gender may be an important consideration in procedure choice and needs further confirmation.
It is notable that about 12% of patients with BMI between 30 and 35 kg/m2 underwent bariatric surgery, particularly in the LAGB group (18% vs 5% in RYGB group). This may reflect patient pressure in the private system, concomitant co-morbidities or pre-operative weight loss prior to the initial consultation, together with the perception that LAGB is reversible and safe. Weight loss in this group of patients was particularly poor (RYGB: -3.9 ± 0.3 kg/m2, and LAGB: -1.5 ± 0.3 kg/m2), highlighting the rigorous protocols for patient selection in order to optimise outcomes.
There are strengths and weaknesses in this current study. The large sample size, a well maintained prospective database and an extended follow-up are the strengths of the current study. To our knowledge, our study is the largest comparative study on RYGB versus LAGB with follow up over 5 years. On the other hand, the weaknesses are potential selection biases by 2 independent surgeons who only performed either RYGB or LAGB, incomplete data relating to plasma biochemistry, and the lack of details on medications for co-morbidities.
In a community setting, RYGB produces substantially greater weight loss and resolution of co-morbidities than LAGB in both the short- and long-term, at a cost of higher peri-operative complications, which are non-fatal and mostly related to wound infection. The long-term complication rate is higher after LAGB. Where band failure occurs, RYGB is the superior salvage procedure. The better outcomes for LAGB in males compared to females after 3 years post-surgery needs further confirmation. Gender, like extent of co-morbidities and BMI, may be an important consideration in procedure choice.
Currently, the choice between these bariatric procedures is based mainly on patient and surgeon preference, and varies significantly between regions of the world. In contrast to the United States and Europe, laparoscopic adjustable gastric band (LAGB) is the most common procedure in Australia and the number of procedures increased by 10 times over the last decade. This is despite the majority of available data indicating that LAGB produces smaller weight loss and less frequent resolution of comorbidities than Roux-en-Y gastric bypass (RYGB).
This study aimed to compare the “real-life” weight loss and surgical outcomes of RYGB and LAGB from two large referral bariatric centres in South Australia, in which the databases were prospectively maintained over 10 years.
In contrast to the impressive weight loss reported by a single Australian LAGB center, the results of the current study are consistent with the available literature on the outcomes of bariatric surgery. The main findings are: (1) RYGB produces substantially greater weight loss and resolution of co-morbidities than LAGB in a community setting, in both the short- and long-term; (2) although peri-operative complications are higher with RYGB than LAGB, which are non-fatal and mostly related to wound infection, the long-term complication rate is higher after LAGB; (3) fasting glucose, total cholesterol and low density lipoprotein levels were significantly lower 12 mo after RYGB than LAGB; and (4) where LAGB fails to induce or maintain weight loss, RYGB appears to be the superior salvage procedure.
This study suggests that, in a community setting, RYGB is safe and produces superior short- and long-term weight loss outcomes than LAGB. These data need to be acknowledged and disseminated in the Australian community to allow both surgeons and patients to make an appropriate decision on the choice of bariatric procedure.
LAGB is a weight loss surgical procedure which involves placement of an inflatable band at the top of the stomach to restrict over-eating. In contrast, RYGB involves surgical reconstruction of both the stomach and the small intestine into a small stomach pouch and a bypass of food through the small intestinal. RYGB not only restricts eating but also leads to malabsorption of food.
This is one of the largest trials that compared the “real-life” weight loss and surgical outcomes of the two most commonly performed bariatric procedures in the world, RYGB and LAGB. The results are interesting and suggest that RYGB is safe in a tertiary centre and is superior to LAGB in term of both short- and long-term weight loss outcomes. The data, therefore, enhance the current knowledge in the area of bariatric surgery and allow appropriate decision making in the management of the epidemic obesity.
P- Reviewers Bayrahtar Y, Marchesini G, Naito Y S- Editor Huang XZ L- Editor A E- Editor Zhang DN
| 1. | Bray GA. Is new hope on the horizon for obesity? Lancet. 2008;372:1859-1860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Yurcisin BM, Gaddor MM, DeMaria EJ. Obesity and bariatric surgery. Clin Chest Med. 2009;30:539-553, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Tice JA, Karliner L, Walsh J, Petersen AJ, Feldman MD. Gastric banding or bypass? A systematic review comparing the two most popular bariatric procedures. Am J Med. 2008;121:885-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 294] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 4. | Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, McManus K, Champagne CM, Bishop LM, Laranjo N. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859-873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1489] [Cited by in RCA: 1232] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 5. | Allison DB, Weber MT. Treatment and prevention of obesity: what works, what doesn’t work, and what might work. Lipids. 2003;38:147-155. [PubMed] |
| 6. | O’Brien PE. Bariatric surgery: mechanisms, indications and outcomes. J Gastroenterol Hepatol. 2010;25:1358-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | O’Brien PE, Brown WA, Dixon JB. Obesity, weight loss and bariatric surgery. Med J Aust. 2005;183:310-314. [PubMed] |
| 8. | Nguyen NT, Slone JA, Nguyen XM, Hartman JS, Hoyt DB. A prospective randomized trial of laparoscopic gastric bypass versus laparoscopic adjustable gastric banding for the treatment of morbid obesity: outcomes, quality of life, and costs. Ann Surg. 2009;250:631-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 9. | Padwal R, Klarenbach S, Wiebe N, Birch D, Karmali S, Manns B, Hazel M, Sharma AM, Tonelli M. Bariatric surgery: a systematic review and network meta-analysis of randomized trials. Obes Rev. 2011;12:602-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 176] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 10. | Romy S, Donadini A, Giusti V, Suter M. Roux-en-Y gastric bypass vs gastric banding for morbid obesity: a case-matched study of 442 patients. Arch Surg. 2012;147:460-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Dixon JB, O’Brien PE. Selecting the optimal patient for LAP-BAND placement. Am J Surg. 2002;184:17S-20S. [PubMed] |
| 12. | O’Brien PE, Dixon JB. Lap-band: outcomes and results. J Laparoendosc Adv Surg Tech A. 2003;13:265-270. [PubMed] |
| 13. | American Society for Metabolic and Bariatric Surgery Clinical Issues Committee. American Society for Metabolic and Bariatric Surgery position statement on global bariatric healthcare. Surg Obes Relat Dis. 2011;7:669-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Mognol P, Chosidow D, Marmuse JP. Laparoscopic gastric bypass versus laparoscopic adjustable gastric banding in the super-obese: a comparative study of 290 patients. Obes Surg. 2005;15:76-81. [PubMed] |
| 15. | Biertho L, Steffen R, Ricklin T, Horber FF, Pomp A, Inabnet WB, Herron D, Gagner M. Laparoscopic gastric bypass versus laparoscopic adjustable gastric banding: a comparative study of 1,200 cases. J Am Coll Surg. 2003;197:536-544; discussion 544-545. [PubMed] |
| 16. | Jan JC, Hong D, Bardaro SJ, July LV, Patterson EJ. Comparative study between laparoscopic adjustable gastric banding and laparoscopic gastric bypass: single-institution, 5-year experience in bariatric surgery. Surg Obes Relat Dis. 2007;3:42-50; discussion 50-51. [PubMed] |
| 17. | Angrisani L, Lorenzo M, Borrelli V. Laparoscopic adjustable gastric banding versus Roux-en-Y gastric bypass: 5-year results of a prospective randomized trial. Surg Obes Relat Dis. 2007;3:127-132; discussion 132-133. [PubMed] |
| 18. | Zuegel NP, Lang RA, Hüttl TP, Gleis M, Ketfi-Jungen M, Rasquin I, Kox M. Complications and outcome after laparoscopic bariatric surgery: LAGB versus LRYGB. Langenbecks Arch Surg. 2012;397:1235-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Ortega E, Morínigo R, Flores L, Moize V, Rios M, Lacy AM, Vidal J. Predictive factors of excess body weight loss 1 year after laparoscopic bariatric surgery. Surg Endosc. 2012;26:1744-1750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 20. | Snyder B, Nguyen A, Scarbourough T, Yu S, Wilson E. Comparison of those who succeed in losing significant excessive weight after bariatric surgery and those who fail. Surg Endosc. 2009;23:2302-2306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Tiwari MM, Goede MR, Reynoso JF, Tsang AW, Oleynikov D, McBride CL. Differences in outcomes of laparoscopic gastric bypass. Surg Obes Relat Dis. 2011;7:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Shen R, Dugay G, Rajaram K, Cabrera I, Siegel N, Ren CJ. Impact of patient follow-up on weight loss after bariatric surgery. Obes Surg. 2004;14:514-519. [PubMed] |
| 23. | Mathus-Vliegen EM. Long-term health and psychosocial outcomes from surgically induced weight loss: results obtained in patients not attending protocolled follow-up visits. Int J Obes (Lond). 2007;31:299-307. [PubMed] |
| 24. | Sarwer DB, Wadden TA, Moore RH, Baker AW, Gibbons LM, Raper SE, Williams NN. Preoperative eating behavior, postoperative dietary adherence, and weight loss after gastric bypass surgery. Surg Obes Relat Dis. 2008;4:640-646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 201] [Article Influence: 11.8] [Reference Citation Analysis (0)] |