Published online Aug 28, 2013. doi: 10.3748/wjg.v19.i32.5326
Revised: July 2, 2013
Accepted: July 17, 2013
Published online: August 28, 2013
Processing time: 105 Days and 7.4 Hours
AIM: To explore the protective effect and the relevant mechanisms of Fufang Biejia Ruangan Pills (FFBJRGP) on hepatic fibrosis in vivo and in vitro.
METHODS: Hepatic fibrosis was induced by carbon tetrachloride composite factors. Adult Wistar rats were randomly divided into four groups: normal control group; hepatic fibrosis model group; FFBJRGP-treated group at a daily dose of 0.55 g/kg; and colchicine-treated group at a daily dose of 0.1 g/kg. The effects of FFBJRGP on liver function, serum levels of hyaluronic acid (HA), type IV collagen (CIV), type III procollagen (PC III), laminin (LN), histopathology, and expression of transforming growth factor (TGF-β1) and Smad3 in hepatic fibrosis were evaluated in vivo. The effects of FFBJRGP on survival rate, hydroxyproline content and cell cycle distribution were further detected in vitro.
RESULTS: Compared with the hepatic fibrosis model group, rats treated with FFBJRGP showed a reduction in hepatic collagen deposition and improvement in hepatic lesions. Compared with those of the model group, the activities of alanine aminotransferase (62.0 ± 23.7 U/L) and aspartate aminotransferase (98.8 ± 40.0 U/L) in the FFBJRGP-treated group were decreased (50.02 ± 3.7 U/L and 57.2 ± 30.0 U/L, respectively, P < 0.01). Compared with those in the model group, the levels of PCIII (35.73 ± 17.90 μg/mL), HA (563.82 ± 335.54 ng/mL), LN (89.57 ± 7.59 ng/mL) and CIV (29.20 ± 6.17 ng/mL) were decreased to 30.18 ± 9.41, 456.18 ± 410.83, 85.46 ± 7.51 and 28.02 ± 9.45 ng/mL, respectively. Reverse-transcriptase polymerase chain reaction and Western blotting also revealed that expression of TGF-β1 and Smad3 were down-regulated in vivo. Cell proliferation was inhibited, the level of hydroxyproline was decreased compared with the control group (P < 0.01), and the cell cycle was redistributed when exposed to FFBJRGP in vitro.
CONCLUSION: FFBJRGP inhibits hepatic fibrosis in vivo and in vitro, which is probably associated with downregulation of fibrogenic signal transduction of the TGF-β-Smad pathway.
Core tip: Fufang Biejia Ruangan Pill (FFBJRGP) is the first anti-fibrosis drug approved by the China State Food and Drug Administration. It has been demonstrated that FFBJRGP has a better efficacy of anti-fibrosis. However, the underlying therapeutic mechanisms of FFBJRGP in hepatic fibrosis are still unclear. In our study, FFBJRGP showed a strong ameliorative effect in hepatic fibrosis in vivo and in vitro. It reduced production and deposition of collagen in liver tissues. FFBJRGP inhibited expression of transforming growth factor (TGF-β1) and Smad3, which implied that inhibition of TGF-β/Smad-mediated fibrogenesis may be a central mechanism by which FFBJRGP protects against liver injury.
-
Citation: Yang FR, Fang BW, Lou JS. Effects of Fufang Biejia Ruangan Pills on hepatic fibrosis
in vivo andin vitro . World J Gastroenterol 2013; 19(32): 5326-5333 - URL: https://www.wjgnet.com/1007-9327/full/v19/i32/5326.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i32.5326
Liver fibrosis represents the final common pathway of virtually all chronic liver diseases. It is characterized by the excessive accumulation of extracellular matrix (ECM) and activated hepatic stellate cells (HSCs) that are undergoing myofibroblast transition. Several studies have shown that hepatic fibrosis is a reversible disease, therefore, an effective treatment would probably prevent or reverse the fibrotic process in the liver[1]. In the long pathological progression of hepatic fibrosis to cirrhosis, transforming growth factor (TGF)-β1 is one of the strongest profibrotic cytokines[2,3], and TGF-β-Smad signaling is the main signal transduction pathway[4], which has been verified by several related studies. The downregulation of TGF-β expression and modulation of TGF-β-Smad signaling may be effective in preventing liver fibrosis[5].
Traditional Chinese medicine plays a unique role in the treatment of liver fibrosis. Fufang Biejia Ruangan Pill (FFBJRGP) has been demonstrated to have a better antifibrotic efficacy for its traditional Chinese medical effects of “softening and resolving hard masses, dissolving blood stasis and detoxication, replenishing Qi and Blood”. Numerous clinical observations have confirmed that patients with hepatic fibrosis receiving FFBJRGP have a favorable outcome[6]. However, the underlying therapeutic mechanisms of FFBJRGP in hepatic fibrosis are still unclear. Thus, in the present study, we investigated the antifibrotic effect and potential mechanisms of action of FFBJRGP in hepatic fibrosis, in order to establish the clinical efficacy and make better application of FFBJRGP.
The composition of FFBJRGP includes Carapax Trionycis, Radix Paeoniae Rubra, Radix Angelicae Sinensis, Codonopsis Pilosula and Radix Astragali.
Animals and experiment protocol: Healthy adult Wistar rats, female and male, weighing 237.8 ± 8.5 g, were obtained from the Experimental Animal Center of Academy of Medical Sciences of Chinese People’s Liberation Army (Beijing, China). All animals were cared for according to the Guide for the Care and Use of Laboratory Animals (NIH Publications, No. 80-23, revised in 1996). Housed in a room with a 12-h light-dark cycle (temperature 22-24 °C and 50%-60% humidity), the rats were given ad libitum access to standard laboratory rodent chow and water. All processes conformed to international guidelines on the ethical use of animals.
The rats were subcutaneously injected with carbon tetrachloride (CCl4) dissolved in peanut oil (CCl4: peanut oil = 4:6, v/v), 0.5 mL/100 g body weight for the first time, and then 0.3 mL/100 g body weight twice weekly for 8 wk. In the first 2 wk, rats were raised with feedstuff I (80% corn meal, 20% lard, and 0.5% cholesterol). After 2 wk, they were raised with feedstuff II (corn meal and 0.5% cholesterol). At the same time, 1 mL 30% alcohol was given orally to each rat every other day from the beginning.
The rats were randomly divided into the normal control group (n = 6); model group (n = 14); FFBJRGP treatment group (n = 12); and colchicine positive control group (n = 12). In the FFBJRGP treatment group, FFBJRGP was administered orally at 0.55 g/kg daily, which was equal to the dose in humans. The rats in the positive control group were given colchicine orally at a daily dose of 0.1 g/kg, which was also equal to the dose for humans. The rats in the normal control and model groups were administered the same volume of physiological saline as for the FFBJRGP group.
Liver laboratory tests: Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities were measured using commercially available kits (Jiancheng Institute of Biotechnology, Nanjing, China) according to the manufacturer’s instructions.
Serum levels of hyaluronic acid, type IV collagen, type III procollagen and laminin: Serum levels of hyaluronic acid (HA), type IV collagen (CIV), type III procollagen (PCIII) and laminin (LN) were determined by radioimmunoassay using commercially available kits (Beifang Institute of Biotechnology, Beijing, China) according to the manufacturer’s instructions.
Liver tissues were collected from the left lobe of the liver of each rat, and fixed in 15% buffered paraformaldehyde, and dehydrated in a graded alcohol series. Specimens were embedded in paraffin blocks, cut into 5-μm-thick sections and placed on glass slides. The sections were stained with hematoxylin-eosin and Ponceau S[7]. Fibrosis was graded according to the method of Scheuer[8] as follows: stage 0: no fibrosis; stage 1: increase in collagen without formation of septa (small satellite expansion of the portal fields), expansion of portal tracts without linkage; stage 2: formation of incomplete septa not interconnecting with each other, from the portal tract to the central vein; stage 3: complete but thin septa interconnecting with each other, which divide the parenchyma into separate fragments; and stage 4: complete cirrhosis, similar to stage 3 with thicker septa. Pathological examination was performed by the same pathologist who was blinded to the treatment assignment for the rats.
Determination of TGF-β1 mRNA level in liver tissues by real-time reverse transcriptase-polymerase chain reaction: Total RNA was extracted from liver tissues of each group with the tissue/cell total RNA isolation kit according to the manufacturer’s protocol (Dalian TaKaRa Biotechnology Company, Dalian, China). The quantity and purity of RNA were detected by determining absorbance at 260/280 nm using a spectrophotometer. Total RNA was reversibly transcribed into cDNA using the cDNA synthesis kit according to the manufacturer’s protocol (Dalian TaKaRa Biotechnology Company, Dalian, China). The ABI PRISM 7900 HT Real Time-polymerase chain reaction (PCR) System and real-time PCR kit were used according to the manufacturers’ instructions. The specific primers for the target gene and β-actin were synthesized by Dalian TaKaRa Biotechnology Company (Dalian, China), as follows: TGF-β1: 5’-TGGCGTTACCTTGGTAACC-3’ (forward); 5’-GGTGTT GAGCCCTTTCCAG-3’ (reverse); β-actin: 5’-ACCCTTAAGGCCAACCGTGA AAAG-3’ (forward); 5’-TCATGAGGTAGTCTGTCAGGT-3’ (reverse).
The two-step PCR procedure was as follows: pre-denaturation for 30 s at 95 °C, 1 cycle; 94 °C for 15 s and 56 °C for 40 s, 40 cycles. The final products were identified by electrophoresis in 1.5% agarose gel and melt curve analysis. Melt curve detection: 95 °C for 15 s, 60 °C for 15 s, and 95 °C for 15 s. The final results were described with the relative values (2-ΔΔCt). The calculation and analysis were performed by Sequence Detection Software version 2.1 in the ABI PRISM 7900 HT Real Time PCR System.
Determination of Smad3 level in liver tissues by Western blotting: Total protein was extracted from liver tissues and analyzed with bicinchoninic acid protein concentration assay kit. Sample protein was separated by electrophoresis in 12% SDS-PAGE with a Bio-Rad electrophoresis system (Hercules, CA, United States). The primary antibodies (rabbit anti Smad3 antibody, 1:1000 dilution) were incubated at 4 °C overnight. The corresponding horseradish-peroxidase-conjugated secondary antibodies (anti-rabbit IgG, 1:5000 dilution) were incubated at room temperature. Immobilon Western chemiluminescent horseradish peroxidase substrate and Quantity ONE were used for revealing and quantitative analysis of the blots. β-actin was used as the internal control.
Drug serum preparation: The normal rats were administered with FFBJRGP and colchicine at a dose of 0.55 and 0.1 g/kg, respectively, for 2 d. At 2 h after the final administration, the sera were collected from the rats, mixed, and inactivated at 56 °C for 30 min. The blank control sera were collected from the normal rats.
Cell culture: HSC-LX-2 cells, an immortalized human HSC line, were cultured in Dulbecco’s Minimal Essential Medium (DMEM) supplemented with 10% fetal bovine serum (FBS). Cultures were placed in a humidified atmosphere of 5% CO2 at 37 °C, and the medium was changed twice a week.
Cell viability test: HSC-LX-2 cells were seeded into 96-well plates at a density of 2 × 104 cells/well until 50% confluence. Cells treated with the above drug sera (20 μL/well) for 48 h were incubated with 5 mg/mL methyl thiazolyl tetrazolium (MTT) in DMEM for 4 h at 37 °C. The supernatant was removed and 100 μL DMSO was added to each well to dissolve the formazan product. Absorbance at 570 nm was measured using a microplate reader.
Determination of hydroxyproline content: Collagen was determined by estimating the hydroxyproline content, an amino acid characteristic of collagen. HSC-LX-2 cells were lysed after treatment with the above drug sera. The lysates were used to measure hydroxyproline content using commercially available kits according to the manufacturer’s instructions (Jiancheng Institute of Biotechnology, Nanjing, China).
Cell cycle analysis: For cell cycle analysis, HSC-LX-2 cells were synchronized by serum starvation in medium containing 0.4% serum for 24 h and induced to re-enter the cell cycle by an exchange of DMEM supplemented with 10% FBS. Drug sera of different groups were added (1 mL/bottle); the cells were cultured for 48 h and then harvested; washed and suspended in phosphate-buffered saline (PBS) twice; fixed in 80% ethanol for 48 h at 4 °C; and suspended in 500 μL PBS containing RNase A for 30 min at 37 °C. A total of 2 × 106 cells were harvested and resuspended in 0.5 mL of a solution containing 50 μg/mL propidium iodide, 1 mg/mL sodium citrate, 100 μg/mL RNase, and 0.1% Triton X-100. Flow cytometric analysis was made with a fluorescence-activated cell sorter. Forward light scatter characteristics were used to exclude cell debris from the analysis. The G0/G1 and S phases of the cell cycle were analyzed by diploid staining profiles.
All values were expressed as mean ± SD. Comparisons were analyzed by one-way ANOVA using the SPSS 12.0 statistical package. Differences were considered statistically significant at P < 0.05.
There were significant differences in the ALT and AST activities among the experimental groups. The ALT and AST activities in the model group were significantly higher compared with those in the normal control group (P < 0.01), while those in the FFBJRGP-treated group (0.55 g/kg) were significantly lower than in the model group (P < 0.01), and those in the colchicine-treated group (0.1 g/kg) were also lower than in the model group (P < 0.05) (Table 1).
| Group | ALT(U/L) | AST(U/L) | PCIII (μg/mL) | HA (ng/mL) | LN (ng/mL) | CIV (ng/mL) |
| Control | 23.8 ± 8.5b | 30.0 ± 11.4b | 15.16 ± 15.12b | 205.30 ± 48.92a | 82.02 ± 8.86 | 21.71 ± 1.76 |
| Model | 62.0 ± 23.7 | 98.8 ± 40.0 | 35.73 ± 17.90 | 563.82 ± 335.54 | 89.57 ± 7.59 | 29.20 ± 6.17 |
| FFBJRGP-treated | 50.02 ± 3.7 | 57.2 ± 30.0b | 30.18 ± 9.41 | 456.18 ± 410.83 | 85.46 ± 7.51 | 28.02 ± 9.45 |
| Colchicine-treated | 46.1 ± 14.8 | 66.0 ± 33.2a | 34.08 ± 9.19 | 313.17 ± 230.06a | 88.61 ± 8.97 | 29.22 ± 7.95 |
The serum levels of PCIII, HA, LN and CIV were significantly increased in the model group, as serum markers of hepatic fibrosis, when compared with the normal control group. The FFBJRGP-treated (0.55 g/kg) and colchicine-treated (0.1 g/kg) groups had decreased serum levels of PCIII, HA, LN and CIV (Table 1).
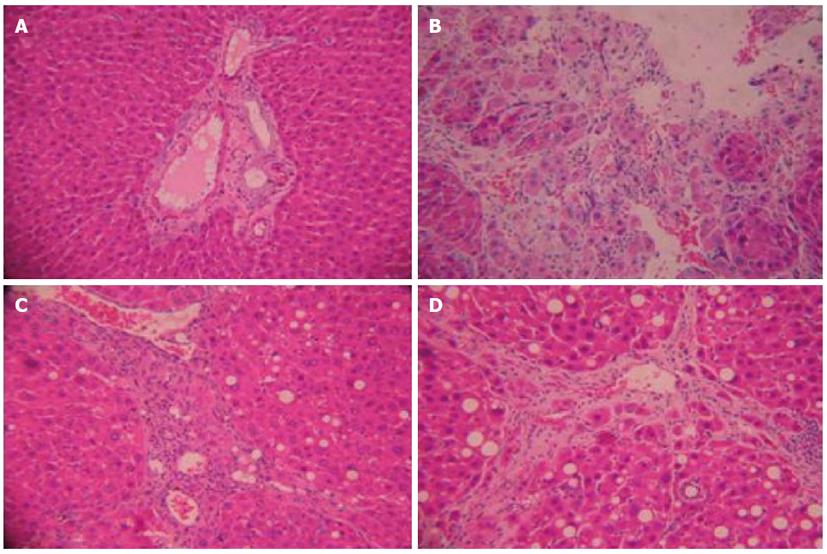
At the end of the study, normal hepatic lobules, without fibroplasia and inflammatory cell infiltration, were observed in normal rats (Figure 1A). Many inflammatory cells infiltrated the intra- and inter-lobular areas, and cell degeneration, focal necrosis and bile duct proliferation were found in rats with hepatic fibrosis (Figure 1B). The histological pattern of the livers treated by FFBJRGP showed a low level of infiltration of leukocytes, necrosis, and bile duct proliferation (Figure 1C). Similar trends were also observed in the colchicine group (Figure 1D).
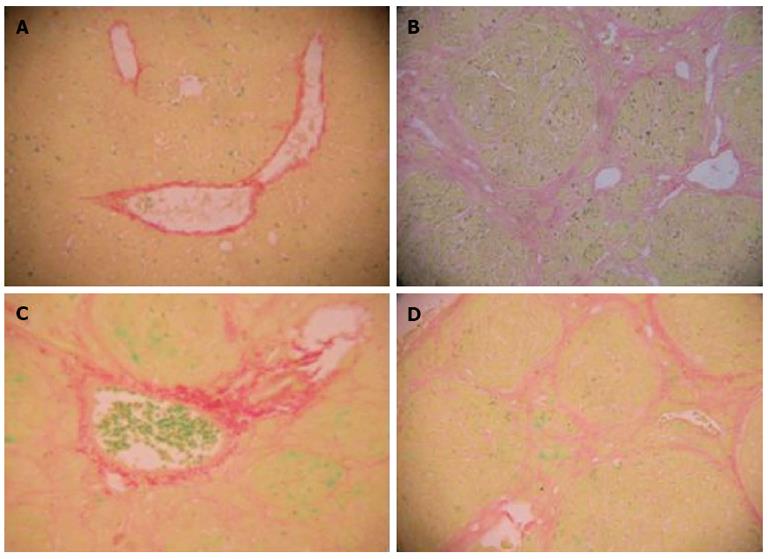
The rat liver was stained with Ponceau S, which showed the collagen fibers as red. Normal hepatic lobules without fibroplasia were observed in normal rats. Complete septa interconnecting with each other were formed, which divided the parenchyma into separate fragments in the model group. The rats treated with FFBJRGP and colchicine had less pronounced destruction of the liver architecture, with decreased collagen deposition (Table 2, Figure 2).
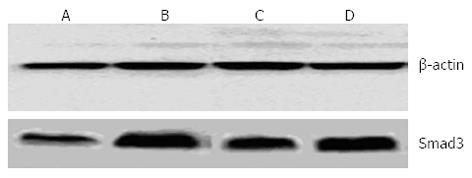
The expression of TGF-β1 and Smad3 in the rat liver was quantified. Expression of TGF-β1 was twofold higher in the model group than in the normal control group. FFBJRGP and colchicine therapy significantly decreased TGF-β1 expression (Table 3). Compared with the normal control group, the expression of Smad3 in the model group was increased (P < 0.01). Compared with the model group, expression of Smad3 was decreased in the FFBJRGP and colchicine groups (Table 3, Figure 3).
The antiproliferative activity in HSC-LX-2 cells was determined by cell viability using the MTT assay. HSC-LX-2 cell proliferation was inhibited by FFBJRGP. Compared with the blank group (100%), FFBJRGP at a dose of 0.55 g/kg inhibited HSC-LX-2 cell proliferation by 31%, and colchicine at a dose of 0.1 g/kg inhibited proliferation by 28%. The antiproliferative effects were not related to the nonspecific cytotoxic effects of FFBJRGP because cells showed normal morphology.
To assess the effect of FFBJRGP on ECM production in HSC-LX-2 cells, hydroxyproline content was examined. Hydroxyproline content was decreased in the FFBJRGP group (1.78 ± 0.06 μg/mL, P < 0.01) compared with the blank group (2.35 ± 0.12 μg/mL), and it was also decreased in the colchicine group (1.91 ±0.14 μg/mL, P < 0.01).
Flow-cytometric assays were carried out to evaluate the effect of FFBJRGP on the cell cycle of activated HSC-LX-2 cells. Compared with the blank control, FFBJRGP altered the percentage of cells in the G0/G1 and S phases. The percentage of cells in the G0/G1 phase was increased in the FFBJRGP group (52.6% ± 1.2%, P < 0.01) compared with the blank group (46.7% ± 0.0%), and the percentage of cells in S phase was decreased in the FFBJRGP group (34.9% ± 7.9%) compared with the blank group (42.1% ± 0.5%). However, the change in the percentage of cells in the G0/G1 and S phases was not obvious in the colchicine group.
Hepatic fibrosis is thought to be a reversible disease, however, at present there is no satisfactory method in clinical practice to reverse the pathological process. Several drugs, including antisense TGF-β receptor, cytokines[9], antioxidants, chemical drugs, soluble type II receptor of TGF-β1, and TGF-β1 antibody have been used to block experimental hepatic fibrosis, but their effects are not as promising as we expected. Besides, some traditional Chinese drugs are effective in preventing fibrogenesis and other causes of chronic liver injury[10], and this offers more hope for the future control of liver fibrosis and cirrhosis[11]. These drugs have the advantages of being cheap, safe and easy to acquire, but most of them are limited in animal experiments and clinical observation, and systematic study at molecular level is lacking.
The activation of HSCs by cytokines is considered to be of importance during the long duration of liver fibrosis. These activated HSCs then become the main source of most cytokines and collagen. Among the cytokine-mediating factors, TGF-β1 is an essential profibrogenic factor[12-17]. In addition, the TGF-β-Smad signaling pathway is the main pathway of TGF-β1[18-20], which transfers the stimulating signal from outside into the affected cells. The Smad proteins consist of a large family of transcription factors, which are also found in vertebrates, insects and nematodes. To date, Smads are the only TGF-β receptor substrates with the ability to propagate signals. Two different transmembrane protein serine/threonine kinases, named as TGF-β receptor type I and II, are brought together by the ligand, which acts as a receptor assembly factor[21]. Before this occurs, receptor I is inactive because a wedge-shaped GS region is inserted into the kinase domain, dislocating the catalytic center. During TGF-β signal transduction, receptor II is activated firstly. TGF-β and its receptor then form an activated complex. In the ligand-induced complex, activated receptor II phosphorylates the GS region of receptor type I, resulting in the activation of the receptor I kinase. The type I receptors specifically recognize the Smad subgroup known as receptor-activated Smads (R-Smads), which are Smad 2 and Smad 3[22]. R-Smads are activated and form a complex consisting of R-Smads and Smad 4, which belongs to Co-Smad. The Smads complex accumulates in the nucleus. This procedure leads to the formation of the functional transcriptional complexes. The R-Smads and Co-Smads in this complex may participate in DNA binding and recruitment of transcriptional cofactors[23]. CREB binding protein is the main downstream molecule and the general transcriptional coactivator. After transfer into the nucleus, the transcriptional complex binds to the certain domain of the target gene and causes gene expression, such as collagen production. Excess collagen production leads to collagen deposition in liver tissues and eventually hepatic fibrosis or cirrhosis. The TGF-β-Smad signaling pathway is important in the formation of hepatic fibrosis, therefore, blocking its transduction may inhibit hepatic fibrosis. Inhibition of the TGF-β-Smad signaling pathway or modulating the gene expression of certain Smads can interfere with hepatic fibrosis[24].
Hepatic fibrosis is characterized by abnormal accumulation of ECM proteins, particularly collagen. The main collagen-producing cells in the liver are HSCs, which proliferate and undergo a process of activation during the development of fibrosis, resulting in increased capacity for collagen synthesis. A simple and reproducible tool is necessary to assess accurately the degree of hepatic fibrosis in clinical practice.
According to the theory of traditional Chinese medicine, hepatic fibrosis is characterized by internal damp (Shi), heat (Re), poison (Du), blood stasis (Yu), and both Qi and Yin asthenia[25,26]. In the present study, not only CCl4, but also cholesterol, lard and alcohol were used to establish a model of hepatic fibrosis. CCl4 is poison, and cholesterol, lard and alcohol produce damp and heat, which cause healthy energy asthenia, blood stasis exacerbation, unrelievable damp and heat, and induce hepatic fibrosis. This model well simulates these symptoms. The serum markers of ECM have been used for the assessment of hepatic fibrosis because they are neither invasive nor unavailable. Serum levels of CIV, PCIII, HA and LN are positively correlated with the inflammatory activity and degree of hepatic fibrosis. Hydroxyproline content in the liver is considered another index of collagen metabolism and provides valuable information about the biochemical and pathological states of liver fibrosis. The present study demonstrated that consumption of FFBJRGP prevented the development of hepatic fibrosis in a rat model of CCl4-induced liver fibrosis. The results were confirmed by both liver histology and quantitative measurement of serum levels of CIV, PCIII, HA and LN. Accordingly, inhibition of proliferation, and reduced collagen content, were also observed in activated HSC-LX-2 cells following FFBJRGP treatment. We also found that FFBJRGP downregulated the expression of TGF-β1 and Smad3, and altered the percentage of cells in the G0/G1 and S phases.
In conclusion, the traditional Chinese medicine FFBJRGP shows significant antifibrotic effects. Inhibiting activation of TGF-β/Smad signaling may be an underlying mechanism by which FFBJRGP protects against chronic liver disease associated with fibrosis.
In China, the incidence of hepatic cirrhosis is still high. Hepatic cirrhosis develops from fibrosis. If treated properly at the fibrosis stage, cirrhosis can be prevented. Fufang Biejia Ruangan Pill (FFBJRGP), a Chinese medical product, is used extensively for the treatment of hepatic fibrosis. FFBJRGP has better antifibrotic efficacy due to its effects of “softening and resolving hard masses, dissolving blood stasis and detoxication, replenishing Qi and Blood” in the philosophy of traditional Chinese medicine. However, the underlying therapeutic mechanisms of FFBJRGP in hepatic fibrosis are still unclear, even though it has become the best-selling traditional Chinese medicine. Thus, in the present study, the authors investigated the antifibrotic effect and potential mechanisms of action of FFBJRGP in hepatic fibrosis, in order to establish the clinical efficacy and make better application of FFBJRGP.
Recent research shows that hepatic fibrosis can be reversed by regulating collagen metabolism, inhibiting the activation of hepatic stellate cells (HSC), or by promoting HSC apoptosis. Hepatic extracellular matrix mainly results from HSCs, which can be activated by the fibrogenesis signaling pathway.
This study confirmed that FFBJRGP can inhibit hepatic fibrosis in vivo and in vitro. FFBJRGP can improve liver function, inhibit collagen deposition, alleviate hepatic injury, inhibit HSC-LX-2 cell proliferation, and redistribute the cell cycle, which is probably associated with its downregulation of the fibrogenic transforming growth factor (TGF)-β-Smad signaling pathway.
FFBJRGP can inhibit hepatic fibrosis in vivo and in vitro, which implies it is a good drug for patients with hepatic fibrosis. This study provides scientific data for its better application.
FFBJRGP is a Chinese medicine that can inhibit hepatic fibrosis. HSCs are key cells that can produce a considerable amount of extracellular matrix and promote collagen deposition. TGF-β1-Smads is a fibrogenic signal transduction pathway that can activate HSCs and promote collagen synthesis.
This study describes the antifibrogenic effects of the Chinese herbal medicine FFBJRGP. The data strongly suggests that FFBJRGP may be therapeutically useful in patients with hepatic fibrosis.
P- Reviewers Butterworth J, Elena V, Jun DW S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Albanis E, Friedman SL. Antifibrotic agents for liver disease. Am J Transplant. 2006;6:12-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. J Cell Mol Med. 2006;10:76-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 538] [Cited by in F6Publishing: 604] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 3. | Li Z, Dranoff JA, Chan EP, Uemura M, Sévigny J, Wells RG. Transforming growth factor-beta and substrate stiffness regulate portal fibroblast activation in culture. Hepatology. 2007;46:1246-1256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 270] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 4. | Parsons CJ, Takashima M, Rippe RA. Molecular mechanisms of hepatic fibrogenesis. J Gastroenterol Hepatol. 2007;22 Suppl 1:S79-S84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 244] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 5. | Prosser CC, Yen RD, Wu J. Molecular therapy for hepatic injury and fibrosis: where are we? World J Gastroenterol. 2006;12:509-515. [PubMed] [Cited in This Article: ] |
| 6. | Gong QM, Xiao JC, Zhou XQ. The clinical study of hepatocirrhosis therapy by using of Fu Fang Bie Jia Ruan Gan Pian. Linchuang Gandanbing Zazhi. 2006;22:196-198. [Cited in This Article: ] |
| 7. | Gong ZJ, Zhan RZ. Common special staining methods. Pathological technique. Shanghai: Shanghai Science and Technology Press 1994; 70. [Cited in This Article: ] |
| 8. | Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13:372-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1130] [Cited by in F6Publishing: 1153] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 9. | Louis H, Le Moine O, Goldman M, Devière J. Modulation of liver injury by interleukin-10. Acta Gastroenterol Belg. 2003;66:7-14. [PubMed] [Cited in This Article: ] |
| 10. | Rockey DC. Antifibrotic therapy in chronic liver disease. Clin Gastroenterol Hepatol. 2005;3:95-107. [PubMed] [Cited in This Article: ] |
| 11. | Zhang G, Zhang FC, Wang TC, Liang KH. [The effects of Chinese national medicine of Huoxueruanjian compound on SMAD signal in hepatic stellate cell and its significance]. Zhonghua Gan Zang Bing Zazhi. 2004;12:213-215. [PubMed] [Cited in This Article: ] |
| 12. | Tahashi Y, Matsuzaki K, Date M, Yoshida K, Furukawa F, Sugano Y, Matsushita M, Himeno Y, Inagaki Y, Inoue K. Differential regulation of TGF-beta signal in hepatic stellate cells between acute and chronic rat liver injury. Hepatology. 2002;35:49-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 155] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Schiller M, Javelaud D, Mauviel A. TGF-beta-induced SMAD signaling and gene regulation: consequences for extracellular matrix remodeling and wound healing. J Dermatol Sci. 2004;35:83-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 311] [Cited by in F6Publishing: 329] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 14. | Gressner AM, Weiskirchen R, Breitkopf K, Dooley S. Roles of TGF-beta in hepatic fibrosis. Front Biosci. 2002;7:d793-d807. [PubMed] [Cited in This Article: ] |
| 15. | Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659-693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1427] [Cited by in F6Publishing: 1494] [Article Influence: 74.7] [Reference Citation Analysis (0)] |
| 16. | Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577-584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 56] [Reference Citation Analysis (0)] |
| 17. | Itoh S, ten Dijke P. Negative regulation of TGF-beta receptor/Smad signal transduction. Curr Opin Cell Biol. 2007;19:176-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 287] [Cited by in F6Publishing: 302] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 18. | Runyan CE, Poncelet AC, Schnaper HW. TGF-beta receptor-binding proteins: complex interactions. Cell Signal. 2006;18:2077-2088. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Xu L. Regulation of Smad activities. Biochim Biophys Acta. 2006;1759:503-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Zhang S, Fei T, Zhang L, Zhang R, Chen F, Ning Y, Han Y, Feng XH, Meng A, Chen YG. Smad7 antagonizes transforming growth factor beta signaling in the nucleus by interfering with functional Smad-DNA complex formation. Mol Cell Biol. 2007;27:4488-4499. [PubMed] [Cited in This Article: ] |
| 21. | Ross S, Hill CS. How the Smads regulate transcription. Int J Biochem Cell Biol. 2008;40:383-408. [PubMed] [Cited in This Article: ] |
| 22. | Hill CS. Identification of a Smad phosphatase. ACS Chem Biol. 2006;1:346-348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Wicks SJ, Grocott T, Haros K, Maillard M, ten Dijke P, Chantry A. Reversible ubiquitination regulates the Smad/TGF-beta signalling pathway. Biochem Soc Trans. 2006;34:761-763. [PubMed] [Cited in This Article: ] |
| 24. | Verrecchia F, Mauviel A. Transforming growth factor-beta signaling through the Smad pathway: role in extracellular matrix gene expression and regulation. J Invest Dermatol. 2002;118:211-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 470] [Cited by in F6Publishing: 501] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 25. | Arias M, Lahme B, Van de Leur E, Gressner AM, Weiskirchen R. Adenoviral delivery of an antisense RNA complementary to the 3’ coding sequence of transforming growth factor-beta1 inhibits fibrogenic activities of hepatic stellate cells. Cell Growth Differ. 2002;13:265-273. [PubMed] [Cited in This Article: ] |
| 26. | Liu P, Hu YY, Ni LQ. [On establishing comparative reference system for syndrome classification study from the thinking characteristics of syndrome differentiation dependent therapy]. Zhongguo Zhong Xi Yi Jie He Zazhi. 2006;26:451-454. [PubMed] [Cited in This Article: ] |