Published online Aug 21, 2013. doi: 10.3748/wjg.v19.i31.5174
Revised: May 29, 2013
Accepted: June 1, 2013
Published online: August 21, 2013
Processing time: 123 Days and 11.5 Hours
The use of herbal products has increased significantly in recent years. Because these products are not subject to regulation by the Food and Drug Administration and are often used without supervision by a healthcare provider, the indication for and consumption of these supplements is quite variable. Moreover, their use is generally regarded as safe and natural by the lay-public. Unfortunately, there has been an increase in the number of reported adverse events occurring with the use of herbal products. We present a case of acute impending liver failure in an adolescent male using a weight-loss product containing green tea extract. Our case adds to the growing concern surrounding the ingestion of green tea extract and serves to heighten healthcare provider awareness of a potential green tea extract hepatotoxicity. Despite the generally touted benefits of green tea as a whole, clinical concern regarding its use is emerging and has been linked to its concentration in multiple herbal supplements. Interestingly, the suspected harmful compounds are those previously proposed to be advantageous for weight-loss, cancer remedy, and anti-inflammatory purposes. Yet, we emphasize the need to be aware of not just green tea extract, but the importance of monitoring patient use of all dietary supplements and herbal products.
Core tip: Green tea extract is one of the most common herbal supplements ingested worldwide and is manufactured into more than 100 different over-the-counter products. Although traditionally considered safe, it has been linked to hepatotoxicity and led to acute impending liver failure in our adolescent patient. Eliminating multiple etiologies and with tissue evidence, a weight-loss supplement containing green tea extract was likely to blame. Recovery was over a two-month course. The lack of regulation and provider guidance in the use of this product and dietary supplements in general is significant. We highlight the importance of monitoring patient use of dietary supplements.
- Citation: Patel SS, Beer S, Kearney DL, Phillips G, Carter BA. Green tea extract: A potential cause of acute liver failure. World J Gastroenterol 2013; 19(31): 5174-5177
- URL: https://www.wjgnet.com/1007-9327/full/v19/i31/5174.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i31.5174
In the United States, herbal products are classified as dietary supplements, and their use has been increasing over recent decades. In fact, the use of herbal medicine increased from 2.5% in the general population in 1990 to 12.1% in 1997, with $5.1 billion spent as out of pocket expenditures for herbal therapies in 1997[1]. Moreover, according to the 2007 National Health Interview Survey, 17.9% of adults reported use of an herbal supplement in the previous year[2]. Yet, as dietary supplements, these products are not subject to the same regulation as drugs approved by the Food and Drug Administration (FDA). Instead, in accordance with the Dietary Supplement Health and Education Act of 1994, dietary supplements do not need approval of their safety or efficacy by the FDA[3].
Green tea has been consumed worldwide for many years and is a popular herbal ingredient that has been manufactured into more than 100 over-the-counter supplements[4]. Green tea’s most touted benefits are its antioxidant and weight-loss or thermogenic properties. Nonetheless, there has been increasing concern regarding the potential hepatotoxicity with the use of green tea extract[5-7]. Here, we present a case of acute impending liver failure in an adolescent male occurring with the use of a weight-loss product containing green tea extract.
Our patient is a 16 year-old Hispanic male, who presented to our emergency room with new onset jaundice. The patient noticed yellowing of his skin and darkening of his urine six to seven days prior to admission. He denied abdominal pain, changes in his stool, fever, changes in mental status, alcohol consumption, sick contacts or recent travel. He is currently a high school student.
He does have a history of obesity and was taking several dietary supplements as part of an unsupervised weight-loss plan. Specifically, he was taking Applied Nutrition® Green Tea Fat Burner beginning 60 d prior to admission and took 2 pills daily (or 400 mg epigallocatechin-3-gallate, EGCG, daily). He started whey protein 30 d prior to admission and mixed 1 scoop in 16.9 oz of water three times per week. In addition, he used GNC Mega Men® Sport, taken 2 pills three times per week, beginning 30 d prior to admission. And lastly, he was taking Nopal® (Cactus), 1 pill daily, beginning 60 d prior to admission. Over this time period, he lost 56 pounds.
On physical exam, the patient was jaundiced, most evident in the face and sclera, but also present on the chest and upper extremities. Mental status was intact. Abdominal exam was insignificant, with the liver difficult to appreciate given that the patient was still overweight at the time of exam. Initial labs included: aspartate aminotransferase (AST) 2106 U/L (normal range 15-40 U/L), alanine aminotransferase (ALT) 2984 U/L (normal range 10-45 U/L), alkaline phosphatase 186 U/L (normal range 116-483 U/L), gamma glutamyl transferase (GGT) 78 U/L (normal range 12-33 U/L), conjugated bilirubin (CB) 12.9 mg/dL (normal range < 0.3 mg/dL), unconjugated bilirubin (UB) 1.9 mg/dL (normal range < 0.1 mg/dL), albumin 4 g/dL (normal range 3.7-5.5 g/dL), partial thromboplastin time 33.9 s (normal range 25.4-34.9 s), protime 15.9 s (normal range 11.2-15.4 s), international normalised ratio (INR) 1.3 (normal range 0.8-1.2), and glucose 99 (Table 1). Thus, he was admitted for work-up of acute liver injury and possible impending liver failure. During his hospitalization his peak INR and CB were 1.5 and 17.5 mg/dL, respectively. His lowest albumin and factor 7 level was 2.5 g/dL and 39% (normal range 58%-150%), respectively, indicating a decline in liver synthetic function and impending liver failure. Radiological exam was done on admission and consisted of an abdominal ultrasound with Doppler, read as mild hepatomegaly with normal right upper quadrant Doppler evaluation.
| Days post-hospitalization | Admission, day 1 | Hospitalized, day 15 | Discharge, day 24 | Follow-up, day 45 | Follow-up, day 94 | Follow-up, day 185 |
| Aspartate aminotransferase (U/L) | 2106 | 958 | 525 | 59 | 33 | 35 |
| Alanine aminotransferase (U/L) | 2984 | 1169 | 665 | 165 | 44 | 31 |
| Alkaline phosphatase (U/L) | 186 | 86 | 137 | 148 | 120 | 94 |
| Gamma glutamyl transferase (U/L) | 78 | 65 | 104 | 49 | 41 | 28 |
| Conjugated bilirubin (mg/dL) | 12.9 | 14.7 | 10.3 | 0.0 | 0.0 | 0.0 |
| Unconjugated bilirubin (mg/dL) | 1.9 | 2.0 | 2.1 | 0.8 | 0.2 | 0.2 |
| Albumin (g/dL) | 4.0 | 2.5 | 3.2 | 3.9 | 4.1 | 4.1 |
| Protime (s) | 15.9 | 18.2 | 14.9 | - | - | - |
| International normalised ratio | 1.3 | 1.5 | 1.2 | 1.0 | 1.0 | 1.0 |
| Factor 7 | - | 42% | - | 102% | - | - |
Extensive lab work was ordered to determine the etiology of his impending liver failure. Serological markers of autoimmune hepatitis (filamentous actin and Liv/Kid antibodies), infectious hepatitis A, B and C (serologies for infectious hepatitis E were not performed given insignificant incidence in the United States), Wilson’s disease (ceruloplasmin), and alpha-1-antitrypsin deficiency were negative. In addition, cytomegalovirus and Epstein-Barr virus immunoglobulin M/immunoglobulin G and adenovirus polymerase chain reaction were negative.
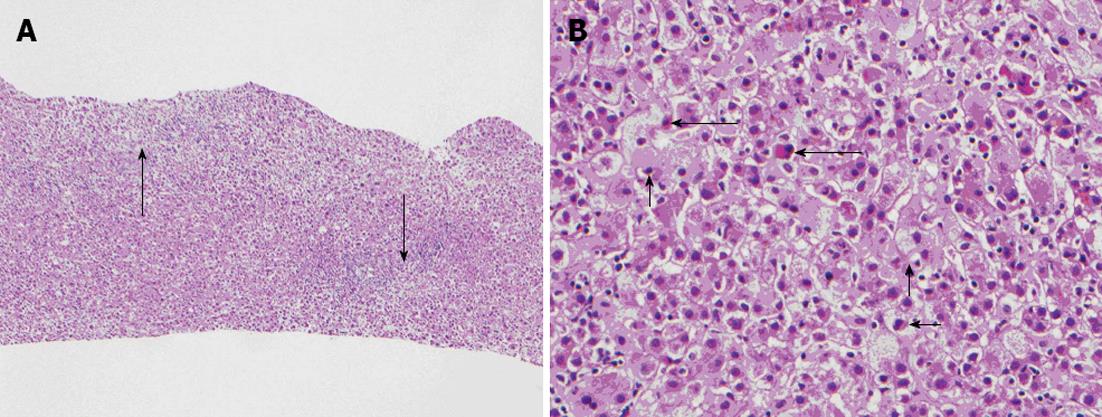
Given lab work as stated, the patient had an ultrasound-guided liver biopsy completed on hospital day 5 (Figure 1). Liver histology was notable for diffuse portal and lobular mixed inflammatory cell infiltrates with acute and chronic inflammation that included scattered eosinophils and interface hepatitis. There was hepatocyte unrest and ballooning degeneration with multifocal individual hepatocyte necrosis and cholestasis. Injury was most prominent in zone 1, but pan-lobular as depicted in Figure 1. He was observed in the hospital until his liver panel began to improve on hospital day twenty-four. Treatment during this admission included initiation of oral vitamin K 5 mg daily on hospital day 2 and ursodiol on hospital day 3. He also received intravenous fluids with a 5% dextrose content, initiated one week after admission and discontinued one week prior to discharge. He was seen again in our clinic at three weeks, ten weeks, and twenty-three weeks after discharge at which time labs (AST, ALT, alkaline phosphatase, GGT, CB, UB, INR and albumin) were repeated. All values continued to improve, along with normalization of both albumin and factor 7 levels, indicating resolution and recovery of his liver function (Table 1).
As several causes of acute liver injury were ruled out, and given his liver histology consistent with previously published reports of toxicity associated with green tea extract, his liver injury can most likely be attributed to his ingestion of this commercially available herbal supplement.
As with our patient, many patients using herbal supplements use a combination of products. Again the use is commonly unsupervised and a deviation from the products’ user instructions. We are associating our patient’s impending liver failure to his ingestion of green tea extract given the history taken, histological findings, and after literature review of all the products and ingredients ingested. A search of the United States National Library of Medicine Dietary Supplements Labels Database and United States National Library of Medicine Clinical and Research Information on Drug Induced Liver Injury for GNC Mega Men® Sport, Nopal® (Cactus), and Whey Protein returned no warnings[8,9]. A PubMed review was significant for a case of acute cholestatic liver injury following ingestion of Whey protein and Creatine supplements. Yet, there have been no other reports that demonstrate this relationship, and instead there have been studies that suggest the hepatoprotective effect of Whey protein in acute and chronic hepatitis[10]. Further investigation of the individual ingredients within the supplements taken, as listed on their respective supplement labels, was concerning for contribution of both Vitamin A and chromium (both contained in the GNC Mega Men® Sport) in development of the liver injury and failure. However, current evidence suggests that Vitamin A toxicity occurs with ingestion of greater than 40000 IU daily or about 12000 micrograms daily[11]. Our patient was taking 5000 IU three times per week, or only 15000 IU per week. In addition, there is no established upper limit of intake of chromium set forth by the Institute of Medicine as there have been few adverse side effects reported[12]. Although likely multi-factorial in nature, current evidence suggests that our patient’s liver outcome is most likely secondary to the green tea extract-containing supplement.
Green tea is made from steaming of the tea plant, Camellia sinensis. Polyphenols, including catechins and flavanols make up 30%-40% of the extractable solid of dried green tea leaves. The main catechins consist of epicatechin, epicatechin-3-gallate, epigallocatechin, and EGCG. It is proposed that these compounds or extracts give green tea its anticarcinogenic, antioxidant, probiotic, and thermogenic properties[13].
Despite studies that show the benefits of green tea, there have been several recent reports that demonstrate hepatotoxicity following the consumption of concentrated green tea extract. Much interest in green tea hepatotoxicity came after the discontinuation of Exolise, a weight-loss product containing a hydroalcoholic extract of green tea, in France and Spain following the report of acute liver injury with the use of this product. The United States Pharmacopeia subsequently reviewed the safety information for green tea products. They found 34 reports of liver damage, ranging from acute hepatitis to fulminant liver failure requiring transplant, following the use of multiple green tea extract preparations[5]. As a result, the United States Pharmacopeia have suggested, but not mandated, a warning, stating symptoms of liver injury be placed on any green tea extract monograph produced[5]. The green tea product ingested by our patient was without such a warning of potential hepatotoxicity.
In reports of green tea extract-associated hepatotoxicity reviewed between 1999 and 2008, histological exam of the livers showed pathology characteristic of inflammatory infiltrates, cholestasis, steatosis, and necrosis[6]. The hepatotoxicity that follows may be attributed to those same compounds within green tea extract that have previously been described as beneficial, and in particular to the catechins, of which EGCG is the most abundant and may be the most potent. The major cytotoxic mechanisms include destruction of mitochondrial membranes and the induction of reactive oxygen species formation[14]. Liver injury typically occurs within three months of ingestion[4].
Thus, although green tea has traditionally been considered safe, emerging reports linking liver injury, and in some cases liver failure, with the use of green tea extract should not be ignored. Several issues remain unresolved, including determination of the preparation types and amounts that can be considered safe versus harmful. This is a difficult task to achieve given the lack of FDA regulation of herbal products and other dietary supplements. There are many supplements that contain various formulations (hydroalcoholic vs aqueous vs powder, etc.) and concentrations of green tea extract in combination with other potentially harmful ingredients. Moreover, there is often inconsistent information regarding the complete list of ingredients contained within dietary supplements. Yet, investigations regarding safety and efficacy of these products are lacking. Resources including the United States National Library of Medicine and The Drug Induced Liver Injury Network account for these adverse events and have been established to help us better understand supplement-related hepatotoxicity[4,7]. Yet, until appropriate standards are established, it is imperative that physicians monitor the use of green tea extract, recognize that it may be contained in a variety of products, and be cognizant of its hepatotoxic potential.
P- Reviewers Elena V, Hashimoto N, Liu QD, Mudawi HMY, Rosenthal P S- Editor Gou SX L- Editor A E- Editor Li JY
| 1. | Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, Van Rompay M, Kessler RC. Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. JAMA. 1998;280:1569-1575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4834] [Cited by in RCA: 4253] [Article Influence: 157.5] [Reference Citation Analysis (0)] |
| 2. | Wu CH, Wang CC, Kennedy J. Changes in herb and dietary supplement use in the U.S. adult population: a comparison of the 2002 and 2007 National Health Interview Surveys. Clin Ther. 2011;33:1749-1758. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 3. | United States Food and Drug Administration. Dietary Supplements. Available from: http://www.fda.gov/Food/DietarySupplements/default.htm. Accessed September 11, 2012. [Cited in This Article: ] |
| 4. | United States National Library of Medicine. Drug Record Green Tea (Camellia sinesis). Available from: http://livertox.nlm.nih.gov/GreenTea.htm. Accessed October 15, 2012. [Cited in This Article: ] |
| 5. | Sarma DN, Barrett ML, Chavez ML, Gardiner P, Ko R, Mahady GB, Marles RJ, Pellicore LS, Giancaspro GI, Low Dog T. Safety of green tea extracts : a systematic review by the US Pharmacopeia. Drug Saf. 2008;31:469-484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in RCA: 206] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 6. | Mazzanti G, Menniti-Ippolito F, Moro PA, Cassetti F, Raschetti R, Santuccio C, Mastrangelo S. Hepatotoxicity from green tea: a review of the literature and two unpublished cases. Eur J Clin Pharmacol. 2009;65:331-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in RCA: 256] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 7. | Stickel F, Kessebohm K, Weimann R, Seitz HK. Review of liver injury associated with dietary supplements. Liver Int. 2011;31:595-605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Available from: http://dietarysupplements.nlm.nih.gov/dietary. Accessed October 15, 2012. [Cited in This Article: ] |
| 9. | Available from: http: //www.livertox.nih.gov/. Accessed October 15, 2012. [Cited in This Article: ] |
| 10. | Whitt KN, Ward SC, Deniz K, Liu L, Odin JA, Qin L. Cholestatic liver injury associated with whey protein and creatine supplements. Semin Liver Dis. 2008;28:226-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | United States National Library of Medicine. Drug Record Vitamin A. Available from: http://livertox.nlm.nih.gov/VitaminARetinoids.htm. Accessed October 15, 2012. [Cited in This Article: ] |
| 12. | Office of Dietary Supplements National Institutes of Health. Dietary Supplement Fact Sheet: Chromium. Available from: http://ods.od.nih.gov/factsheets/Chromium-HealthProfessional/. Accessed October 15, 2012. [Cited in This Article: ] |
| 13. | Green tea. Altern Med Rev. 2000;5:372-375. [PubMed] [Cited in This Article: ] |
| 14. | Galati G, Lin A, Sultan AM, O’Brien PJ. Cellular and in vivo hepatotoxicity caused by green tea phenolic acids and catechins. Free Radic Biol Med. 2006;40:570-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in RCA: 252] [Article Influence: 13.3] [Reference Citation Analysis (0)] |