Published online Aug 21, 2013. doi: 10.3748/wjg.v19.i31.5076
Revised: June 28, 2013
Accepted: July 9, 2013
Published online: August 21, 2013
Processing time: 177 Days and 21.3 Hours
AIM: To investigate the capacity of shunts to relieve portal hypertension and decrease the safe minimal liver remnant in pigs.
METHODS: A subtotal hepatectomy with < 60 mL blood loss and without hepatic pedicle occlusion was performed. The mesenteric venous inflow was diverted through a mesocaval shunt (MCS) constructed using the prepared left renal vein with an end-to-side running suture of 5-0 proline. All 21 animals that underwent subtotal hepatectomy and/or MCS were divided into three groups. In the 15% group, the residual volume was 14%-19% of total liver volume (TLV); in the 15%+ S group, the residual volume was also 14%-19% of TLV with a mesocaval shunt (MCS); and in the 10%+ S group, the residual volume was 8%-13% of TLV with an MCS. In the three groups, the intraoperative portal vein pressure (PVP) and portal vein flow (PVF) were monitored and compared at laparotomy and 1 h post-hepatectomy. The survival rate, sinusoidal endothelial damage, tissue analysis, and serum analysis were investigated among the three groups.
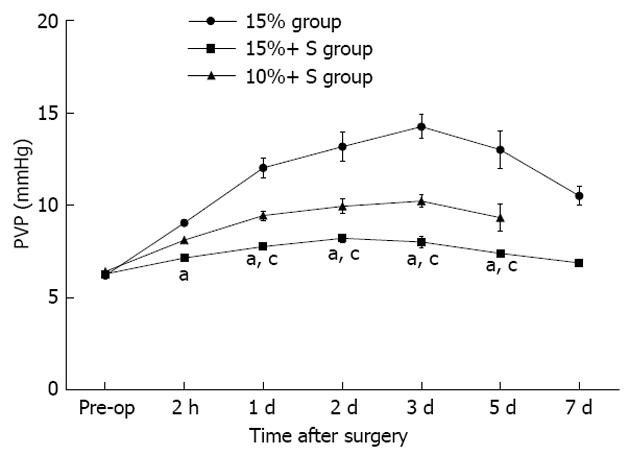
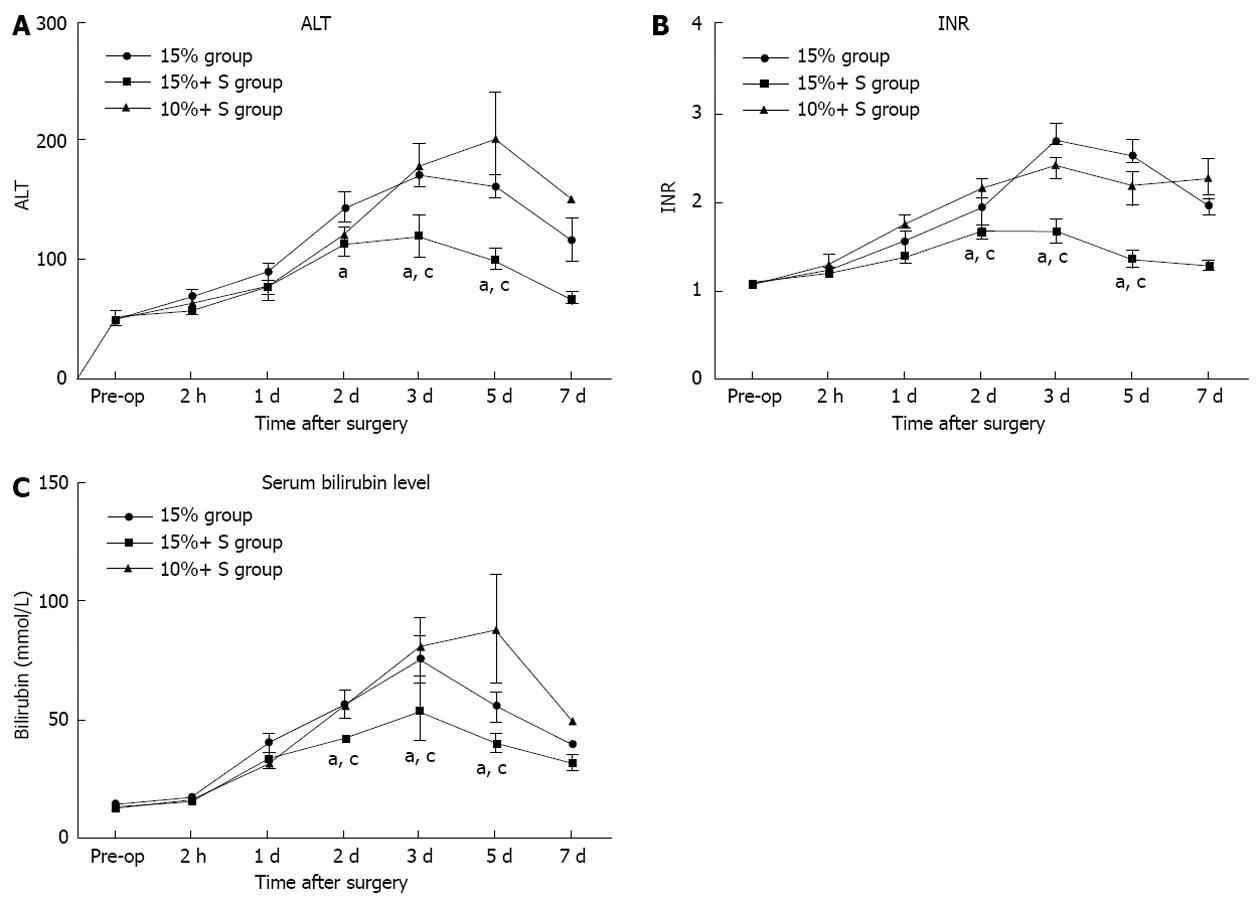
RESULTS: The percentage residual liver volume was 15.9%, 16.1% and 11.8% in the 15%, 15%+ S, 10%+ S groups, respectively. After hepatectomy, PVF and portal-to-arterial flow ratio in the 15%+ S group significantly decreased and hepatic artery flow (HAF) per unit volume significantly increased, compared to those in the 15% group. The PVP in the 15%+ S group and 10%+ S group increased slightly from that measured at laparotomy; however, in the 15% group, the PVP increased immediately and significantly above that observed in the other two groups. The 14-d survival rates were 28.5%, 85.6%, and 14.2% in the 15%, 15%+ S, and 10%+ S groups, respectively. In the 15%+ S group, the shunts effectively attenuated injury to the sinusoidal endothelium, and the changes in the serum and tissue analysis results were significantly reduced compared to those in the 15% and 10%+ S groups.
CONCLUSION: MCS can decompress the portal vein and so attenuate liver injury from hyperperfusion, and make extreme or marginal hepatectomy safer.
Core tip: When the residual liver volume is extremely small after extended hepatectomy or living-donor liver transplantation, postoperative hepatic failure (PHF) or small-for-size syndrome (SFSS) may result from portal hypertension or hyperperfusion. We demonstrated that mesocaval shunt attenuated portal overflow injury, however, it is unknown how much the shunt can decrease, and whether the shunt can do the same for small liver remnants following subtotal hepatectomy. We showed that the residual volume was the determinant factor of PHF or SFSS after subtotal hepatectomy, and the shunt attenuated injury from hyperperfusion, and made marginal hepatectomy safer.
- Citation: Tu YL, Wang X, Wang DD, Zhu ZM, Tan JW. Impact of mesocaval shunt on safe minimal liver remnant: Porcine model. World J Gastroenterol 2013; 19(31): 5076-5084
- URL: https://www.wjgnet.com/1007-9327/full/v19/i31/5076.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i31.5076
Currently, there is no definitive answer to the question “How much liver excision is too much?”[1-4]. When the residual liver volume or graft is extremely small after extended hepatectomy or living-donor liver transplantation (LDLT), postoperative hepatic failure (PHF) or small-for-size syndrome (SFSS) may ensue, and portal hypertension or hyperperfusion is regarded as the determinant factor of liver failure or SFSS. It has been demonstrated the portal decompression, such as portacaval or mesocaval shunt (PCS/MCS), splenic artery ligation or splenectomy, can attenuate portal overflow injury, and result in smaller graft or liver remnant generated successfully in animal experiments and clinical studies[5-8]. However, it is unknown how much the shunt can decrease portal overflow, and whether the shunt can do the same for small liver remnants following subtotal hepatectomy[7]. Large-animal models provide a clinically relevant means of investigating the pathophysiology of a disease process that can be more readily applied in the human setting[9-11]. We investigated the capacity of MCS to relieve sinusoidal microcirculatory injury and to decrease the safe minimal liver remnant (MLR) value in massive hepatectomy.
Twenty-five male Bama miniature pigs (15-20 kg) were obtained from the Pig and Poultry Production Institute (Guangxi Province, China). The pigs were raised from a closed herd and kept under strict quarantine. The study was approved by the Chinese PLA General Hospital Clinic Committee on Ethics in Animal Experimentation. All animals in this study were treated humanely and in accordance with institutional and national guidelines for ethical animal experimentation.
The pigs were food-deprived for 8 h before the operation. All pigs were anesthetized in the following way: initial sedation was obtained with a deep intramuscular injection of ketamine (15-20 mg/kg) and chlorpromazine (6-8 mg/kg) 15 min after atropine (0.01 mg/kg). Oxygen saturation and heart rate were monitored throughout the operation. A size 4 laryngeal mask airway was inserted, and anesthesia was maintained using 1.5% halothane in oxygen titrated to provide anesthesia. Central venous access was established with a tunneled catheter from the right femoral vein. Intraoperatively, 1 L of normal saline and 500 mL 5% dextrose were administered intravenously. No attempt was made to lower central venous pressure.
An upper-midline incision with right or bilateral subcostal extensions (inverse “L” shape or Mercedes incision) was performed. A subtotal hepatectomy with < 60 mL blood loss and without hepatic pedicle occlusion was performed as previously described described[12-14]. A 16-gauge catheter was inserted into the main portal vein via the gastroduodenal vein to measure the portal vein pressure (PVP). Another catheter was advanced into the suprahepatic inferior vena cava (IVC) through one of the phrenic veins to monitor the pressure in the IVC. Ultrasonic flow probes were connected to a flow meter (TS420; Transonic Systems, Ithaca, NY, United States) to measure hepatic artery flow (HAF) and portal vein flow (PVF).
The mesenteric venous inflow was diverted through an MCS constructed using the prepared left renal vein with an end-to-side running suture of 5-0 proline (Qiangsheng, Shanghai, China), while the mesenteric vein was partly occluded (Figure 1). After the shunt, its patency was examined, and the size of shunt was adjusted to preserve PVF.
After the operation, the pigs were monitored for 14 d: every 2 h in the first day and every 24 h thereafter, and one dose of 375 mg penicillin/375 mg streptomycin was given intramuscularly to all pigs. This dose was repeated daily every morning until euthanasia. They were given free access to water. Food and water intake and serum glucose levels were evaluated at each postoperative assessment, and animals that had limited or no intake per os and/or low serum glucose levels (< 70 mg/dL) were administered 50 g intravenous glucose (500 mL 10% glucose solution). Every dead or euthanized pig was necropsied to examine the patency of the shunt.
Twenty-five pigs were included and four were excluded for the obliteration of MCS or other surgery-related complications. Based on previous studies[13,14], the remaining 21 animals, which were submitted to massive hepatectomy with different liver mass removed, were divided into three groups: 15% group (n = 7), which was submitted to massive hepatectomy with a residual volume of approximately 15% (range 14%-19%, median: 15.9%) of TLV (Table 1); 15%+ S group (n = 7), which was subjected to MCS (Figure 1) and then massive hepatectomy with a residual volume of approximately 15% (range 14%-19%, median: 16.1%) of TLV; 10%+ S group (n = 7), with the same surgical procedure as the 15%+ S group, but with a residual volume of approximately 10% (range 8%-13%, median: 11.2%) of TLV (Table 1). In the 15%+ S group and 10%+ S group, there was a portal inflow of 3.0-3.5 times baseline per unit volume, which was regarded as a optimum flow for liver regeneration based on our previous study and other studies[15]. This was maintained through regulating the size of the MCS after hepatectomy. In the three groups, the intraoperative PVP and PVF were monitored and compared at laparotomy and 1 h post-hepatectomy (PH). The survival rate and tissue and serum analysis among the three groups were also investigated.
| 15% group | 15%+ S group | 10%+ S group | 1P value | 2P value | |
| Body weight (kg) | 19.6 ± 2.9 | 18.4 ± 3.0 | 18.9 ± 3.9 | NS | NS |
| Left-trilobes (g) | 347.51 ± 18.2 | 334.6 ± 16.4 | 340.7 ± 17.2 | NS | NS |
| ETL (g) | 434.4 ± 22.5 | 418.1 ± 21.4 | 425.8 ± 21.2 | NS | NS |
| WRL (g) | 362.1 ± 17.3 | 355.0 ± 16.6 | 368.1 ± 16.9 | - | - |
| ERL (g) | 69.3 ± 4.5 | 67.8 ± 4.8 | 47.7 ± 3.1 | - | - |
| Rate of RL (%) | 15.9 | 16.1 | 11.8 | - | - |
| PVF, mL/min per 100 g | |||||
| BAS | 61.3 ± 7.1 | 62.9 ± 5.9 | 59.1 ± 4.3 | NS | NS |
| PH | 312.4 ± 24.1 | 215.4 ± 20.3 | 231.4 ± 31.2 | 0.001 | NS |
| HAF, mL/min per 100 g | |||||
| BAS | 21.1 ± 4.6 | 19.4 ± 4.5 | 18.6 ± 3.4 | NS | NS |
| PH | 8.3 ± 3.4 | 15.5 ± 4.1 | 14.1 ± 3.4 | 0.001 | NS |
| P/A | |||||
| BAS | 2.9 ± 0.3 | 3.2 ± 0.4 | 3.3 ± 0.3 | NS | NS |
| PH | 36.3 ± 4.1 | 14.1 ± 2.6 | 16.4 ± 3.6 | 0.000 | NS |
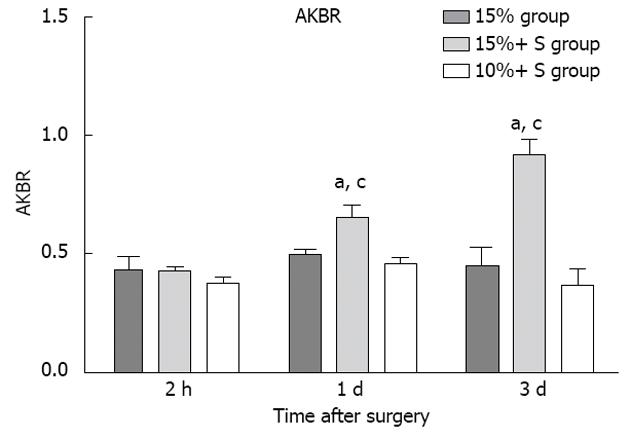
Blood sampling was performed preoperatively, and 1 h PH, then daily for 7 d or until death. During the follow-up period, levels of alanine aminotransferase (ALT) and total bilirubin (TB) and the international normalized ratio (INR) were determined. Hyaluronic acid (HA) is a polysaccharide synthesized by mesenchymal cells and eliminated chiefly by receptor-mediated endocytosis in the hepatic sinusoidal endothelium, and increased serum HA levels reflect sinusoidal endothelial damage[16]. HA was measured by a radiometric assay with the Pharmacia HA test (Yihua BioScience, Shanghai, China) in pre-reperfusion and post-reperfusion serum samples. The arterial ketone body ratio (acetoacetate/β-hydroxybutyrate, AKBR) is a useful tool for the estimation of liver functional reserve. Liver mitochondrial redox state (liver mitochondrial free NAD+/NADH ratio), which indicates hepatic energy charge, is known to reflect the ketone body ratio (acetoacetate/3-hydroxybutyrate) in liver tissue[15,17]. Ozawa et al[17] first demonstrated that the AKBR was correlated with the ketone body ratio in liver tissue, and it has been reported as a useful tool for the estimation of liver functional reserve in hepatic surgery. The AKBR was measured preoperatively and at 2 h and 48 h PH.
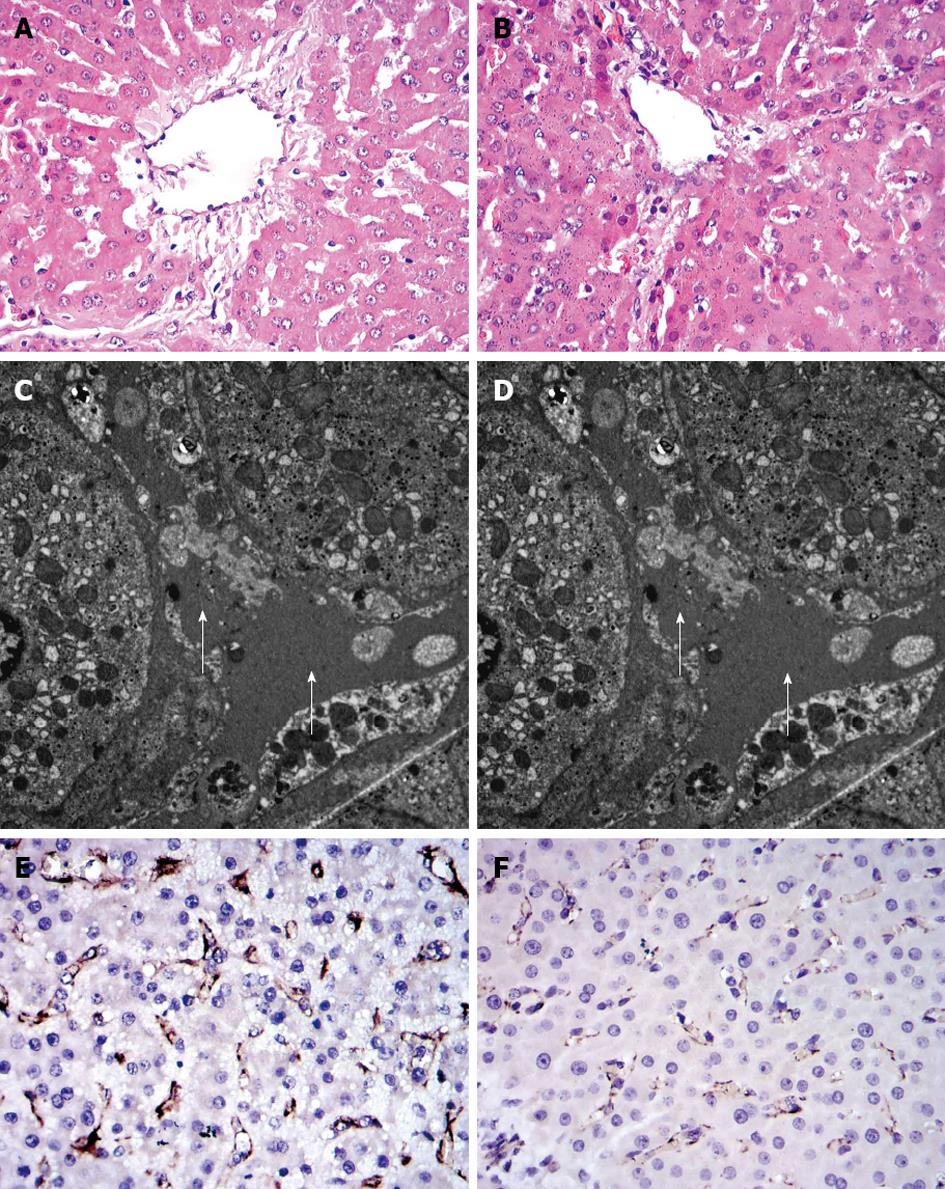
Hepatic tissue specimens were obtained from the edges of the liver at laparotomy and from the edges of the remnant liver at 2 h PH, and then divided into two sections. One was preserved in 10% formaldehyde for subsequent fixation in paraffin, and the other was immediately cut into 1-mm cubes and fixed in 2.5% glutaraldehyde in cacodylate buffer (0.1 mol/L sodium cacodylate-HCl buffer, pH 7.4) overnight at 4 °C prior to sectioning for transmission electron microscopy to study hepatocyte and sinusoidal ultrastructure. Platelet endothelial cell adhesion molecule-1 (CD31) helps maintain endothelial stability by interacting with other CD31 molecules at the extracellular border of adjacent cells. Sections of hepatic tissue were immunostained with porcine anti-CD31 antibody (Serotec, Oxford, United Kingdom) to evaluate the integrity of the endothelial cells in the hepatic sinusoid, as previously described[18].
The lipopolysaccharides (LPS) level was quantitated by a limulus amebocyte lysate (LAL) assay based on the methods first introduced by Iwanaga et al[19] using the commercially available chromogenic LAL Endpoint Kit (Yihua BioScience, Shanghai, China) following the manufacturer’s instructions. A calculated value of 0.1 EU/mL (10 pg/mL) was considered the threshold for LPS positivity. Standards and samples were analyzed in duplicate. Serum levels of tumor necrosis factor (TNF)-α and interleukin (IL)-6 were measured using commercial ELISA kits (Jingmei Biotech Co. Ltd., Shenzhen, China) following the manufacturer’s instructions.
The survival rates in the three groups were calculated using a generalized Wilcoxon test. The biochemical results were compared by Student’s t test, comparing mean values among the three groups. Parameters are presented as mean ± SD. Statistical significance was determined by Student’s t test (SPSS, Chicago, IL, United States). P < 0.05 was regarded as significant.
The characteristics of the study and the evolution of hemodynamic parameters are shown in Table 1. The percentage RLV was 15.9%, 16.1%, and 11.8% in the 15%, 15%+ S, and 10%+ S groups, respectively. After hepatectomy, PVF and portal-to-arterial flow ratio in the 15%+ S group significantly decreased and HAF per unit volume significantly increased, compared to those in the 15% group.
Serial changes in PVP in the three groups are shown in Figure 2. PVP in the 15%+ S and 10%+ S groups increased slightly from that measured at laparotomy; however, in the 15% group, PVP increased immediately and significantly compared to that observed in the other two groups (P < 0.05 for all comparisons).
Changes in HA concentration are shown in Figure 3. Two hours after subtotal hepatectomy, serum HA concentration increased in all pigs. In the 15%+ S group, HA level was significantly reduced compared to that in the 15% group. At other time points, the values were significantly lower than those observed in the 15% and 10%+ S groups (P < 0.01). The histological changes in tissue samples taken at 1 h PH in the three groups are shown in Figure 4.
The serial measurements of serum ALT, TB, and INR are shown in Figure 5, in which significant differences were noted. There were significant differences between the 15% and 15%+ S groups, and between the 10%+ S and 15%+ S groups (P < 0.05 for two comparisons).
The animals were followed-up for 14 d. An observation period of 14 d was chosen because liver function recovered to normal within 14 d after major hepatectomy[20]. The survival rate was calculated by the Kaplan-Meier method. Survival in the 15%+ S group with a shunt was better than in the 15% and 10%+ S groups (85.7% vs 28.5% vs 14.3%, P < 0.01). In the 15% group without a shunt, all pigs survived for > 4 d, and only two pigs survived until 14 d. In the 10%+ S group, all pigs survived > 3 d, but only one pig survived until 14 d.
In hepatectomy, when the RLV decreases below a certain threshold, the liver vascular bed immediately decreases, and vascular resistance in the residual liver increases. This leads to portal hypertension or hyperperfusion and a steady decrease in liver function; the liver remnant cannot sustain metabolic, synthetic, and detoxifying functions; and SFSS or PLF ensues[1,3]. The portal hypertension or hyperperfusion is regarded as the determinant factor of liver failure or SFSS. Portal decompression, such as PCS/MCS, splenic artery ligation or splenectomy, is often used to improve the prognosis of an SFSS graft in LDLT when the graft-to-recipient weight ratio is < 0.8% or the graft volume/standard liver volume (GV/SLV) is < 30%[5-7,21-23]. PCS/MCS could make a GV/SLV < 30%, or as low as 20%-25% a viable option with a fair prognosis[24-26], indicating shunts can make small grafts successfully regenerate or make them safe. However, it is unknown how much the shunt can decrease portal pressure, and whether the shunt can do the same for small liver remnants following subtotal hepatectomy as it does in LDLT[7].
In our previous study, we showed that the survival rate in pigs with approximately 15% residual liver volume was 24.8%, which was similar to the present study; whereas it was 100% in pigs with approximately 20% RLV. We established that the safe MLR should be > 15% of TLV in a porcine model. However, in the present study, we found that MCS could decrease the degree of sinusoidal injury, protect liver function, regenerate liver with approximately 15% RLV, and increase the survival rate up to 85.7%. Nonetheless, none of the pigs with 10% RLV in the 10%+ S group could sustain metabolism and failed to regenerate even though portal decompression was performed. These data also indicate that portal decompression can make extreme liver resection or marginal size liver remnant (approximately 15% of TLV) safe, but it cannot make LRV < 5% (10% of TLV) viable.
In the normal state, PVF and HAF are linked by the hepatic artery buffer response[27,28], which induces a decrease in hepatic artery diameter and flow if PVF increases, and is synonymous with liver microcirculation failure. In the present study, it also showed the HAF in the 15% group was significantly decreased (Table 1), and this insufficient HAF might be another important contributor to the failure of liver remnant regeneration. However, the MCS prevented injury from excess PVF and significantly decreased PVF, resulting in a significant increase in HAF in the 15%+ S group; successfully regenerated liver in animals with approximately 15% RLV; but it could not regenerate 10% RLV. This is probably due to the liver remnant being too small to sustain the metabolic, synthetic and detoxifying functions, despite the presence of sufficient arterial flow. AKBR is a predictor of liver viability and responds to disorders of energy metabolism in the mitochondria[16]. The present study also demonstrated that there was no significant difference among the three groups at 2 h PH. At other time points, the 15%+ S group showed significant differences from the 15% and 10%+ S groups (P < 0.05), indicating the optimum portal inflow and safe MLR in the 15%+ S group were important for recovery of liver energy metabolism (Figure 6).
However, MCS was a “double-edged sword”. Excessive diversion of portal flow results in a portal pressure that is insufficient to promote liver regeneration[24,25,29,30]. It is well known that vascular shear stress in the portal vein is a major determinant factor of regeneration[25]. Therefore, diversion by MCS should be controlled. Hessheimer et al[29] demonstrated twice-baseline portal inflow was necessary for the functional recovery of a small-for-size liver graft. In the present study, the portal flow was preserved at approximately 3.2 times baseline to avoid portal hypoperfusion and benefit liver remnant regeneration. The portal flow was similar to that in 70% hepatectomy model which was supposed to be the optimum portal flow for liver regeneration[31]. It was also indentified that a portal inflow 3.2 times the baseline value can greatly stimulate the regeneration of the liver remnant without causing hyperperfusion injury.
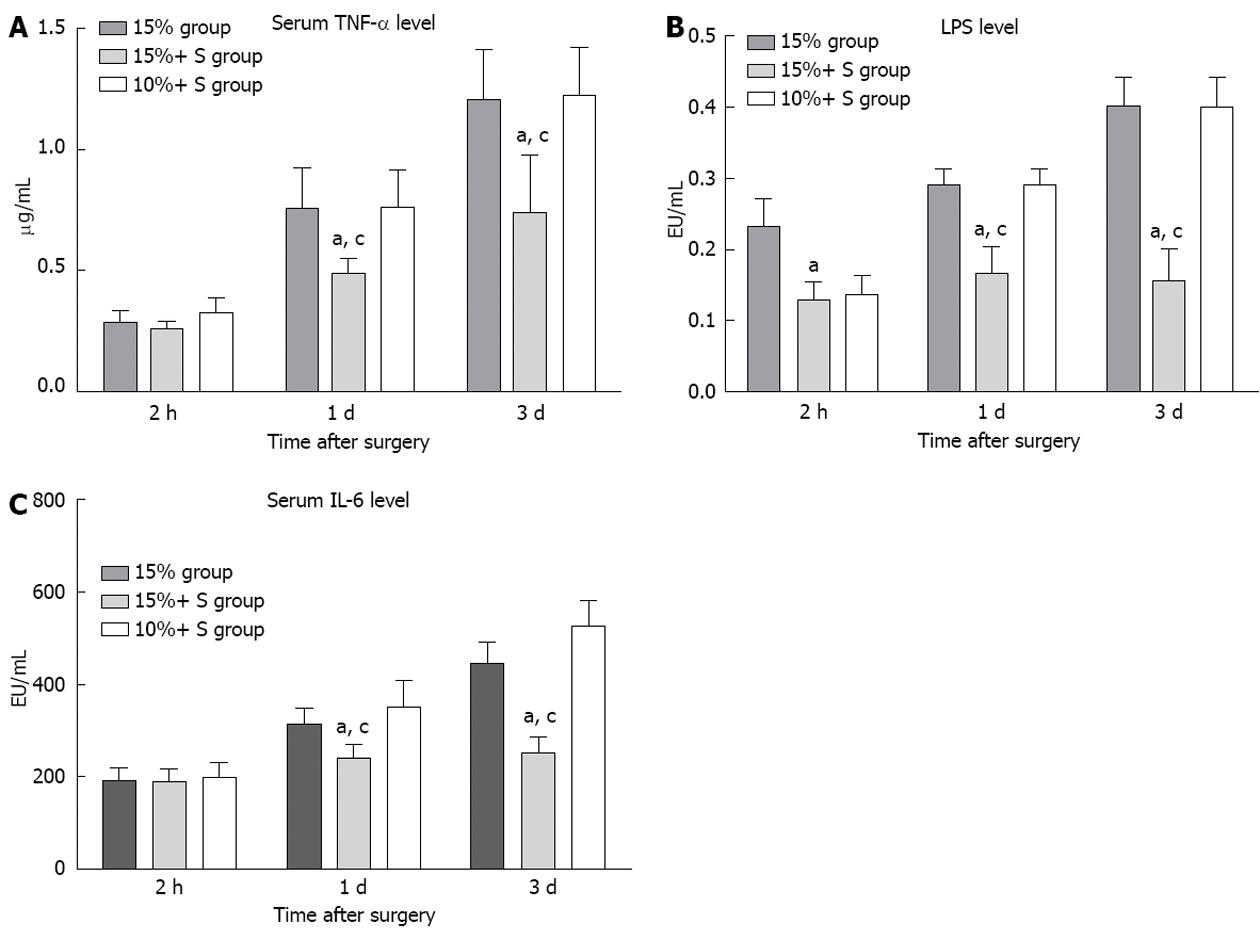
In addition, the hepatic parenchyma contains an abundance of reticuloendothelial cells; after subtotal resection, the reticuloendothelial function declines, and portal hyperperfusion further promotes endotoxin absorption and bacterial translocation. Bacterial infection and bacteremia are serious complications that are frequently encountered in patients with subtotal hepatectomy. In this study, severe endotoxin or bacterial translocation in the 15% group was significantly elevated compared to the 15%+ S group, and serum TNF-α or IL-1 level was significantly elevated (Figure 7), also indicating that optimum portal decompression relieves portal overflow injury, and decreases the endotoxin/bacterial translocation[32], which play important roles in delaying liver remnant regeneration.
In summary, the decompression of portal vein can decrease the hyper-reperfusion injury, and make the marginal size hepatectomy safer. Therefore, the portal decompression modality should be considered when the risk of PHF or SFSS in hepatectomy is high or one-stage resection is adopted for the small future residual liver volume, for which portal venous embolism or two-stage resection is usually adopted.
Currently, there is no definitive answer to the question “How much liver excision is too much?”. When the residual liver volume or graft is extremely small after extended hepatectomy or living-donor liver transplantation, postoperative hepatic failure (PHF) or small-for-size syndrome (SFSS) may ensue, and portal hypertension or hyperperfusion is regarded as the determinant factor of liver failure or SFSS.
The authors demonstrated that mesocaval shunt could attenuate portal overflow injury, however, it is unknown how much the shunt can decrease, and whether the shunt can do the same for small liver remnants following subtotal hepatectomy.
The authors showed that portal vein decompression decreased hyper-reperfusion injury, and made “marginal size” hepatectomy safer, but did not reduce the safe value of the minimal residual volume (MRV) to < 5% of TLV.
The portal decompression modality should be considered when the risk of PHF or SFSS in hepatectomy is high, or one-stage resection is adopted for small future residual liver volume, in which portal venous embolism or two-stage resection is usually adopted.
This study demonstrated that portal vein decompression decreased hyper-reperfusion injury, and made marginal size hepatectomy safer, but did not reduce the safe value of the MRV to < 5% of TLV. Therefore, the portal decompression modality should be considered when the risk of PHF or SFSS in hepatectomy is high, or one-stage resection is adopted for small future remnant liver volume.
P- Reviewers Bulbuloglu E, Ramalho FS S- Editor Wen LL L- Editor Kerr C E- Editor Ma S
| 1. | Vauthey JN, Chaoui A, Do KA, Bilimoria MM, Fenstermacher MJ, Charnsangavej C, Hicks M, Alsfasser G, Lauwers G, Hawkins IF. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000;127:512-519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 494] [Cited by in F6Publishing: 471] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 2. | Shoup M, Gonen M, D’Angelica M, Jarnagin WR, DeMatteo RP, Schwartz LH, Tuorto S, Blumgart LH, Fong Y. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003;7:325-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 350] [Cited by in F6Publishing: 327] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 3. | Kiuchi T, Tanaka K. How much liver does the patient need? Split Liver Transplantation: Theoretical and Practical Aspects. Darmstadt: Springer 2002; 105–114. [DOI] [Cited in This Article: ] |
| 4. | Morioka D, Egawa H, Kasahara M, Ito T, Haga H, Takada Y, Shimada H, Tanaka K. Outcomes of adult-to-adult living donor liver transplantation: a single institution’s experience with 335 consecutive cases. Ann Surg. 2007;245:315-325. [PubMed] [Cited in This Article: ] |
| 5. | Lo CM, Fan ST, Chan JK, Wei W, Lo RJ, Lai CL. Minimum graft volume for successful adult-to-adult living donor liver transplantation for fulminant hepatic failure. Transplantation. 1996;62:696-698. [PubMed] [Cited in This Article: ] |
| 6. | Tanaka K, Yamada T. Living donor liver transplantation in Japan and Kyoto University: what can we learn? J Hepatol. 2005;42:25-28. [PubMed] [Cited in This Article: ] |
| 7. | Imura S, Shimada M, Ikegami T, Morine Y, Kanemura H. Strategies for improving the outcomes of small-for-size grafts in adult-to-adult living-donor liver transplantation. J Hepatobiliary Pancreat Surg. 2008;15:102-110. [PubMed] [Cited in This Article: ] |
| 8. | Botha JF, Langnas AN, Campos BD, Grant WJ, Freise CE, Ascher NL, Mercer DF, Roberts JP. Left lobe adult-to-adult living donor liver transplantation: small grafts and hemiportocaval shunts in the prevention of small-for-size syndrome. Liver Transpl. 2010;16:649-657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 9. | Campos BD, Botha JF. Strategies to optimize donor safety with smaller grafts for adult-to-adult living donor liver transplantation. Curr Opin Organ Transplant. 2012;17:230-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Wilms C, Mueller L, Lenk C, Wittkugel O, Helmke K, Krupski-Berdien G, Rogiers X, Broering DC. Comparative study of portal vein embolization versus portal vein ligation for induction of hypertrophy of the future liver remnant using a mini-pig model. Ann Surg. 2008;247:825-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Loos M, Friess H. Is there new hope for patients with marginally resectable liver malignancies. World J Gastrointest Surg. 2012;4:163-165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Abulkhir A, Limongelli P, Healey AJ, Damrah O, Tait P, Jackson J, Habib N, Jiao LR. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008;247:49-57. [PubMed] [Cited in This Article: ] |
| 13. | Court FG, Laws PE, Morrison CP, Teague BD, Metcalfe MS, Wemyss-Holden SA, Dennison AR, Maddern GJ. Subtotal hepatectomy: a porcine model for the study of liver regeneration. J Surg Res. 2004;116:181-186. [PubMed] [Cited in This Article: ] |
| 14. | Wang H, Ohkohchi N, Enomoto Y, Usuda M, Miyagi S, Masuoka H, Sekiguchi S, Kawagishi N, Fujimori K, Sato A. Effect of portocaval shunt on residual extreme small liver after extended hepatectomy in porcine. World J Surg. 2006;30:2014-2022; discussion 2023-2024. [PubMed] [Cited in This Article: ] |
| 15. | Yamaoka Y, Washida M, Manaka D, Gubernatis G, Ringe B, Ozaki N, Yamaguchi T, Takada Y, Ollerich M, Ozawa K. Arterial ketone body ratio as a predictor of donor liver viability in human liver transplantation. Transplantation. 1993;55:92-95. [PubMed] [Cited in This Article: ] |
| 16. | Itasaka H, Suehiro T, Wakiyama S, Yanaga K, Shimada M, Sugimachi K. Significance of hyaluronic acid for evaluation of hepatic endothelial cell damage after cold preservation/reperfusion. J Surg Res. 1995;59:589-595. [PubMed] [Cited in This Article: ] |
| 17. | Ozawa K, Aoyama H, Yasuda K, Shimahara Y, Nakatani T, Tanaka J, Yamamoto M, Kamiyama Y, Tobe T. Metabolic abnormalities associated with postoperative organ failure. A redox theory. Arch Surg. 1983;118:1245-1251. [PubMed] [Cited in This Article: ] |
| 18. | Newman PJ. The biology of PECAM-1. J Clin Invest. 1997;99:3-8. [PubMed] [Cited in This Article: ] |
| 19. | Iwanaga S, Morita T, Harada T, Nakamura S, Niwa M, Takada K, Kimura T, Sakakibara S. Chromogenic substrates for horseshoe crab clotting enzyme. Its application for the assay of bacterial endotoxins. Haemostasis. 1978;7:183-188. [PubMed] [Cited in This Article: ] |
| 20. | Nagino M, Ando M, Kamiya J, Uesaka K, Sano T, Nimura Y. Liver regeneration after major hepatectomy for biliary cancer. Br J Surg. 2001;88:1084-1091. [PubMed] [Cited in This Article: ] |
| 21. | Schindl MJ, Redhead DN, Fearon KC, Garden OJ, Wigmore SJ. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54:289-296. [PubMed] [Cited in This Article: ] |
| 22. | Yigitler C, Farges O, Kianmanesh R, Regimbeau JM, Abdalla EK, Belghiti J. The small remnant liver after major liver resection: how common and how relevant? Liver Transpl. 2003;9:S18-S25. [PubMed] [Cited in This Article: ] |
| 23. | Truant S, Oberlin O, Sergent G, Lebuffe G, Gambiez L, Ernst O, Pruvot FR. Remnant liver volume to body weight ratio & gt; or =0.5%: A new cut-off to estimate postoperative risks after extended resection in noncirrhotic liver. J Am Coll Surg. 2007;204:22-33. [PubMed] [Cited in This Article: ] |
| 24. | Troisi R, Ricciardi S, Smeets P, Petrovic M, Van Maele G, Colle I, Van Vlierberghe H, de Hemptinne B. Effects of hemi-portocaval shunts for inflow modulation on the outcome of small-for-size grafts in living donor liver transplantation. Am J Transplant. 2005;5:1397-1404. [PubMed] [Cited in This Article: ] |
| 25. | Suehiro T, Shimada M, Kishikawa K, Shimura T, Soejima Y, Yoshizumi T, Hashimoto K, Mochida Y, Hashimoto S, Maehara Y. Effect of intraportal infusion to improve small for size graft injury in living donor adult liver transplantation. Transpl Int. 2005;18:923-928. [PubMed] [Cited in This Article: ] |
| 26. | Lo CM, Liu CL, Fan ST. Portal hyperperfusion injury as the cause of primary nonfunction in a small-for-size liver graft-successful treatment with splenic artery ligation. Liver Transpl. 2003;9:626-628. [PubMed] [Cited in This Article: ] |
| 27. | Rocheleau B, Ethier C, Houle R, Huet PM, Bilodeau M. Hepatic artery buffer response following left portal vein ligation: its role in liver tissue homeostasis. Am J Physiol. 1999;277:G1000-G1007. [PubMed] [Cited in This Article: ] |
| 28. | Richter S, Mücke I, Menger MD, Vollmar B. Impact of intrinsic blood flow regulation in cirrhosis: maintenance of hepatic arterial buffer response. Am J Physiol Gastrointest Liver Physiol. 2000;279:G454-G462. [PubMed] [Cited in This Article: ] |
| 29. | Hessheimer AJ, Fondevila C, Taurá P, Muñoz J, Sánchez O, Fuster J, Rimola A, García-Valdecasas JC. Decompression of the portal bed and twice-baseline portal inflow are necessary for the functional recovery of a “small-for-size” graft. Ann Surg. 2011;253:1201-1210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Fondevila C, Hessheimer AJ, Taurá P, Sánchez O, Calatayud D, de Riva N, Muñoz J, Fuster J, Rimola A, García-Valdecasas JC. Portal hyperperfusion: mechanism of injury and stimulus for regeneration in porcine small-for-size transplantation. Liver Transpl. 2010;16:364-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 31. | Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol. 2010;176:2-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 503] [Cited by in F6Publishing: 548] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 32. | Yagi S, Iida T, Hori T, Taniguchi K, Nagahama M, Isaji S, Uemoto S. Effect of portal haemodynamics on liver graft and intestinal mucosa after small-for-size liver transplantation in swine. Eur Surg Res. 2012;48:163-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |