Published online Jan 21, 2013. doi: 10.3748/wjg.v19.i3.404
Revised: December 7, 2012
Accepted: December 15, 2012
Published online: January 21, 2013
AIM: To assess the diagnostic value of using magnifying chromoendoscopy combined with immunohistochemical staining of proliferating cell nuclear antigen (PCNA) and p53 in the detection of gastric precancerous lesions.
METHODS: Ninety-five patients who were treated for abdominal discomfort, abdominal pain, bloating, and acid reflux at our hospital from January 2010 to December 2011 were included in the study. An ordinary gastroscopic procedure was initially performed to select the lesions. All subjects underwent magnifying chromoendoscopy to observe morphological changes of gastric pits. Biopsies were then taken from each area of interest and sent for pathological examination and detection of PCNA and p53 expression by immunohistochemistry. An immunoreactivity score for each lesion was calculated. Based on immunoreactivity scores, immunohistochemical staining was then considered.
RESULTS: Compared to intestinal metaplasia, gastric pits were more diverse in size, more irregular in shape, and more disorderly in arrangement in moderate and severe dysplasia. PCNA and p53 expression was significantly higher in precancerous lesions (intestinal metaplasia and dysplasia) than in chronic gastritis. PCNA expression showed an upward trend in types A-F pits. The number of cases that showed strong PCNA positivity increased significantly with an increase in the severity of lesions. Rank sum test for independent samples showed that p53 expression was significantly higher in types E and F pits than in types A-D pits (H = 33.068, P = 0.000). Rank sum test for independent samples showed that PCNA expression was significantly higher in types E and F pits than in types A-D pits (H = 31.791, P = 0.001).
CONCLUSION: The presence of types E and F pits, in which p53 and PCNA are highly expressed, is highly suggestive of the occurrence of early cancer, and patients developing these changes should be closely followed.
- Citation: Meng XM, Zhou Y, Dang T, Tian XY, Kong J. Magnifying chromoendoscopy combined with immunohistochemical staining for early diagnosis of gastric cancer. World J Gastroenterol 2013; 19(3): 404-410
- URL: https://www.wjgnet.com/1007-9327/full/v19/i3/404.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i3.404
Gastric cancer is one of the most common human malignancies, ranking first in terms of both incidence and mortality among gastrointestinal malignancies in China. Although great technological progress has been made in the diagnosis and treatment of gastric cancer, there is no significant improvement in its prognosis, and the 5-year survival rate of gastric cancer is only about 20%[1]. This is because 67% of patients have already had local spread or distant metastasis at initial diagnosis[1]. Therefore, early diagnosis and treatment are important for improving the prognosis of gastric cancer.
Early gastric cancer only invades the mucosal layer and submucosal layer and has a 5-year survival rate as high as 90% to 95%. Gastroscopy has the advantages of easy operation and low false-negative detection rate and has long been considered the preferred and most reliable diagnostic modality for gastric cancer. It allows detection of minute lesions and flat lesions easily, estimation of the depth of invasion[2], and the performance of histological and molecular biological analysis of diseased tissue; it therefore has a higher detection rate of early cancer and precancerous lesions. Thus, it is important to carry out a regular follow-up of gastric precancerous lesions to increase the detection rate of early gastric cancer. However, the widespread use of gastroscopy does not significantly improve the detection rate of early gastric cancer. A recent study in Japan[3] indicates that endoscopy has a sensitivity of only 81% in detecting early gastric cancer, and this means that the false-negative rate is 19%. Therefore, worldwide efforts are being made to improve the sensitivity of endoscopy in the detection of early cancer and precancerous lesions so that the tumor can be detected at an early treatable stage.
The human genome is composed of about 100 000 genes, and the selective expression of these genes determines the whole human life course. Changes in gene expression play a central role in biological regulatory mechanisms. Tumorigenesis is the result of multiple genetic changes. Activation of oncogenes and inactivation or deletion of tumor suppressor genes are considered closely related to tumorigenesis. The tumor suppressor gene p53 is well known as the guardian of the cell. To date, extensive and in-depth research on p53 has been conducted. Wild-type p53 is an important negative regulator of cell growth and can antagonize oncogenic functions in vivo to maintain the relative stability between positive and negative proliferation signals. Wild-type p53 is involved in many important biological processes such as cell cycle, DNA repair, cell differentiation, and apoptosis.
Proliferating cell nuclear antigen (PCNA) is an acidic nuclear protein that is involved in eukaryotic DNA synthesis and plays an important role in the regulation of the cell cycle. PCNA expression is significantly up-regulated in the process of malignant transformation of normal epithelium and is deemed a marker reflecting cell proliferation activity in most tumor types.
The aim of this study was to assess the diagnostic value of magnifying chromoendoscopy combined with detection of PCNA and p53 in the detection of gastric precancerous lesions.
Ninety-five patients who were treated for abdominal discomfort, abdominal pain, bloating, and acid reflux at our hospital from January 2010 to December 2011 were selected. There were 60 males and 35 females. Their ages ranged from 17 to 78 years, with a median age of 52 years.
Endoscopic examination: The procedure was performed using a Fujinon (EG-590) gastroscope and EPX 4400
processor. An ordinary gastroscopic procedure was initially performed to select the lesions, and magnifying chromoendoscopy was then performed to observe morphological changes of gastric pits. Three to four biopsies were taken from each area of interest and sent for pathological examination and detection of PCNA and p53 expression.
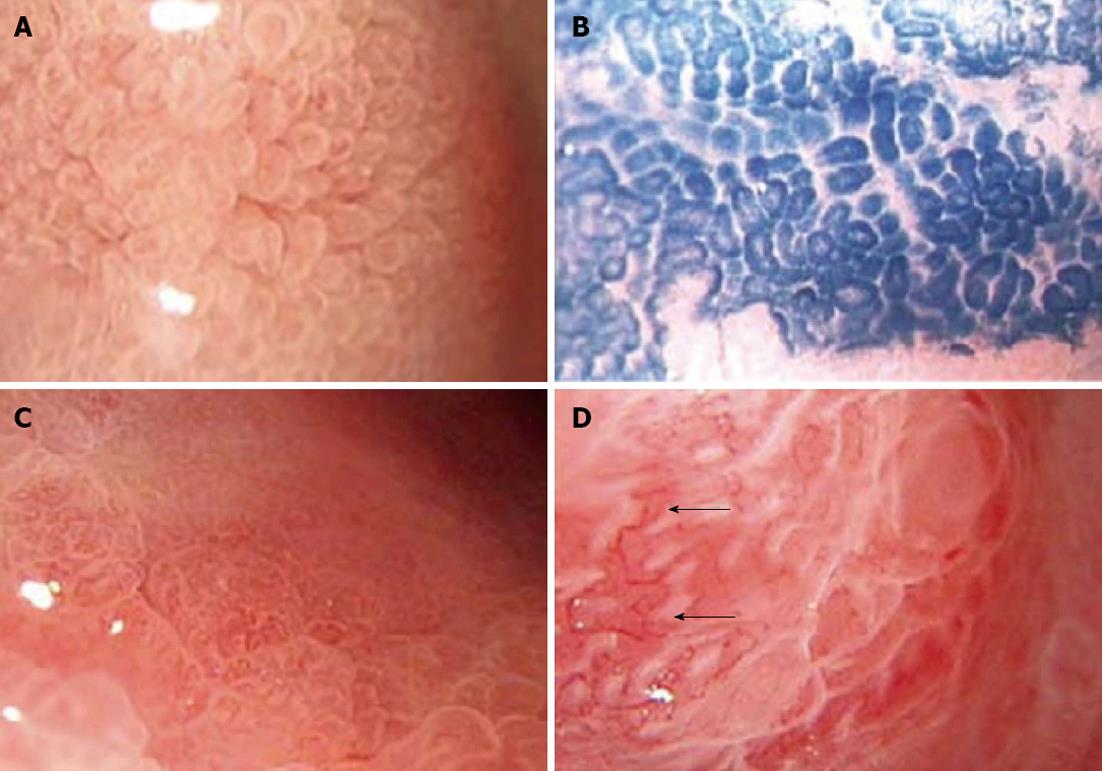
Criteria for morphological classification: Based on morphological classification criteria proposed by Yang et al[4], gastric pits were divided into six fundamental types: A (round spot-like pits), B (short rod-like pits, arranged regularly and tightly, with elongations, branches and curvatures), C (linear pits, more sparse and thick compared to type B), D (patchy pits, elongated and tortuous pits connected to form a reticular appearance), E (villous pits, with finger-like tubers similar to enteral villus-like changes) and F (the pits have obscure or disappearing structures and extremely irregular arrangement). The boundaries between bowl-shaped defects on the erosion surface and neighboring structures are unclear. Defect areas may show granular protrusions and irregular thickened capillaries). When two or more types of gastric pits were present in a lesion, the lesion was defined as having the severe type of pits.
Immunohistochemical staining for PCNA and p53: Immunohistochemistry: Immunohistochemical analysis was performed using the streptavidin-peroxidase method to determine the expression of p53 and PCNA in the gastric mucosa. Briefly, tissue samples were fixed in 10% neutral buffered formalin, dehydrated in a graded series of ethanol, cleared, paraffinized, and cut into 4 μm sections. The sections were deparaffinized, hydrated in a graded series of ethanol, incubated in a microwave to heat and high pressure cook for 10 min for antigen retrieval, and washed in tris-buffered saline (TBS) (3 × 5 min). After treatment with 3% H2O2 for 10 min, slides were washed again in TBS (3 × 5 min). The slides were then incubated with primary anti-PCNA (1:50; Maxim, Fuzhou, China) or anti-p53 (1:100; Maxim) antibody for 30 min at room temperature and washed in TBS (3 × 5 min). Immunolabeling was visualized by reaction with DAB for 10 min at room temperature. The sections were counterstained with hematoxylin, dehydrated and mounted. This experiment was performed by an experienced technician.
Evaluation of immunohistochemical staining: Scoring of immunohistochemical results was performed according to the semi-quantitative method. The percentage of immunopositive cells was scored using a four-point system as follows: 0 point, < 5% of positive cells; 1 point, 5%-25% of positive cells; 2 points, 26%-50% of positive cells; 3 points, 51%-75% of positive cells; 4 points, > 75% of positive cells. The staining intensity was scored similarly, with 0 point for negative staining, 1 point for weak staining (light yellow), 2 points for moderate staining (brown), and 3 points for strong staining (dark brown). Immunoreactivity score for each lesion was calculated as (the score for the percentage of immunopositivity cells + the score for the staining intensity)/2. Based on immunoreactivity scores, immunohistochemical staining was considered negative (< 0.5), weakly positive (0.5-1.5), or strongly positive (> 1.5). Estimation of scores for all lesions was performed by the same pathologist.
All statistical analysis were performed using SPSS 11.5 software. P values ≤ 0.05 were considered statistically significant.
Relation between gastric pit patterns and histopathological phenotypes: A total of 248 biopsies were taken under a magnifying chromoendoscope. Of these biopsies, 9 had type A gastric pits, 38 had type B, 42 had type C, 67 had type D, 62 had type E, and 30 had type F (Table 1).
| Pit type | Case | CSG | CAG | IM | Dysplasia | |
| Mild | Severe | |||||
| A | 9 | 8 | 1 | 0 | 0 | 0 |
| B | 38 | 32 | 6 | 0 | 0 | 0 |
| C | 42 | 35 | 7 | 0 | 0 | 0 |
| D | 67 | 9 | 56 | 7 | 0 | 0 |
| E | 62 | 2 | 6 | 50 | 3 | 1 |
| F | 30 | 0 | 1 | 3 | 20 | 6 |
| Total | 248 | 74 | 84 | 60 | 23 | 7 |
Under a magnifying chromoendoscope, intestinal metaplasia was characterized by the presence of type E gastric pits (villous pits). Dysplasia also showed changes similar to those of intestinal metaplasia (e.g., the presence of villous, linear or patchy pits); however, some characteristic changes were observed in some cases of dysplasia. Compared to intestinal metaplasia, gastric pits were more diverse in size, more irregular in shape, and more disorderly in arrangement in moderate and severe dysplasia. Type F gastric pits in the 30 cases of dysplasia showed varying degrees of these changes (Figure 1).
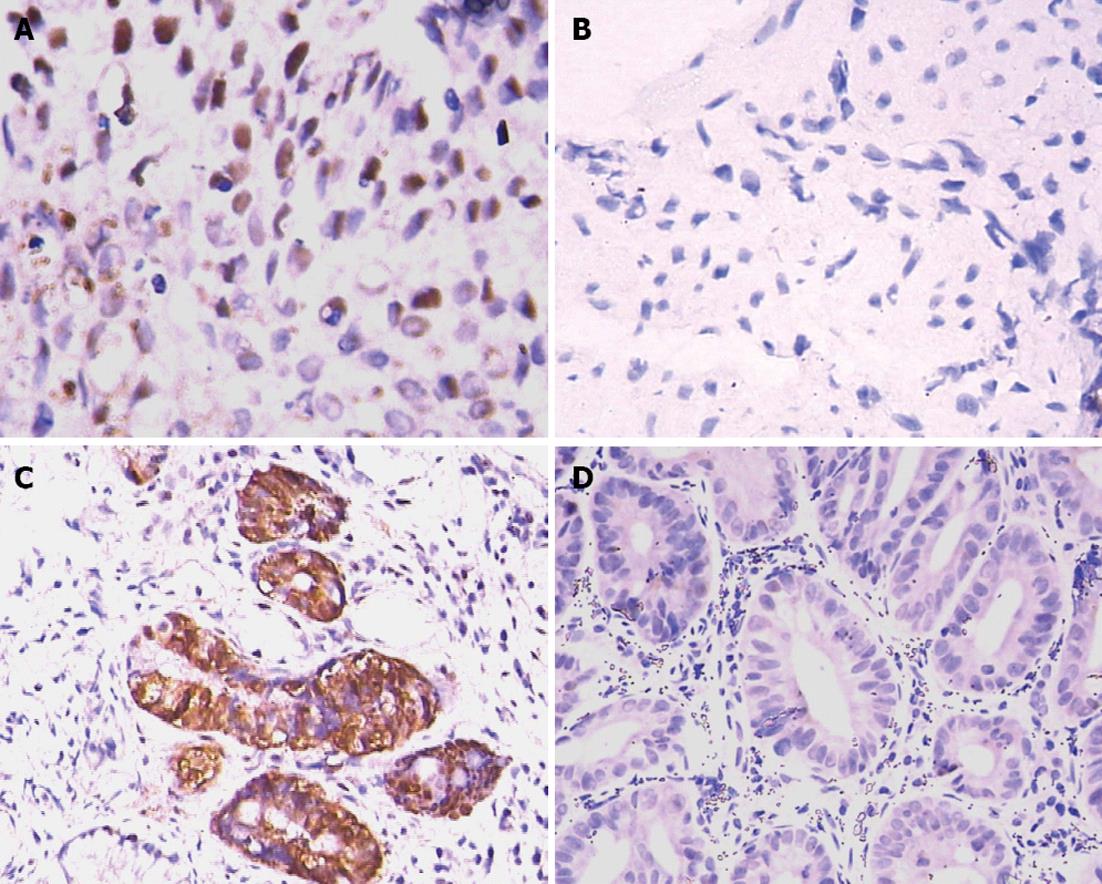
p53 expression: p53 expression was lowest in type A gastric pits and highest in type F pits, showing an upward trend from types A to F pits. p53 expression intensity also showed an upward trend (Figure 2A and B). Rank sum test for independent samples showed that p53 expression was significantly higher in types E and F pits than in types A-D pits (H = 33.068, P = 0.000). Between-group comparisons showed that p53 expression was significantly higher in types E and F pits than in type A pits (Table 2).
| Group | Cases | p53 expression | Positive cases | PCNA expression | Positive cases | ||||
| - | + | ++ | - | + | ++ | ||||
| A | 9 | 7 | 2 | 0 | 2 (22.2) | 6 | 3 | 0 | 3 (33.3) |
| B | 38 | 21 | 14 | 3 | 17 (44.7) | 23 | 12 | 3 | 15 (39.7) |
| C | 42 | 21 | 18 | 3 | 21 (50.0) | 16 | 21 | 5 | 26 (61.9) |
| D | 67 | 28 | 35 | 4 | 39 (58.2) | 22 | 35 | 10 | 45 (67.2) |
| E | 62 | 18 | 38 | 6 | 44 (71.0) | 14 | 35 | 13 | 48 (77.1) |
| F | 30 | 4 | 19 | 7 | 26 (86.7) | 2 | 13 | 15 | 28 (93.3) |
PCNA expression: PCNA expression was lowest in type A gastric pits and highest in type F pits, showing an upward trend from types A to F pits (Figure 2C and D). The number of cases that showed strong PCNA positivity increased significantly with an increase in the severity of lesions. Rank sum test for independent samples showed that PCNA expression was significantly higher in types E and F pits than in types A-D pits (H = 31.791, P = 0.001). Between-group comparisons showed that PCNA expression was significantly higher in types E and F pits than in type A pits (Table 2).
The normal gastric mucosa surface is divided by crisscross small grooves into many lesser gastric areas, in which there are many spot- or rod-like gastric pits. When gastric mucosal lesions occur, morphological changes of gastric pits often appear first. Under an ordinary microscope, it is difficult to observe the morphological changes of gastric pits. In contrast, a magnifying endoscope has a magnification comparable to that of a stereomicroscope and allows observing gastric pit patterns clearly. Dye staining is an important auxiliary method for magnifying endoscopy. Magnifying chromoendoscopy can more clearly show the extent and surface conditions of lesions, which is conducive to clearer observation of lesions and more accurate biopsy. In recent years, domestic and foreign scholars have divided gastric mucosal pit patterns into four fundamental types: spot-like, reticular, granular, and villous[4,5]. Yang et al[4] and Huang et al[6] discovered that gastric pit morphology correlates with the severity of mucosal inflammation. They further divided gastric pits into five fundamental types: A (round spot-like pits), B (short rod-like pits), C (elongated and tortuous pits), D (reticular pits), and E (villus-like pits). This classification can more accurately reflect the evolution from the normal mucosa to superficial gastritis and atrophic gastritis. The presence of type E pits is a characteristic change of intestinal metaplasia. Wang et al[7] believe that epithelial and gland hyperplasia and intestinal metaplasia are main factors leading to morphological changes of gastric pits in atrophic gastritis. Endo et al[8] examined intestinal metaplasia of Barrett’s esophagus and gastric cardia and found that villus-like pits are a characteristic change of intestinal metaplasia.
Gastric mucosal erosions are common lesions that show a special pattern during endoscopy, and some of them have malignant potential with the progression of inflammation. Therefore, it is particularly important to detect and determine the nature of mucosal erosions by endoscopy. By carefully observing gastric erosions, we found that the evolution of six fundamental types of gastric pits reflects the evolution from the normal mucosa to superficial gastritis, atrophic gastritis, intestinal metaplasia, and dysplasia. Type F pits evolve gradually from type E pits. They have obscure or disappearing structures and an extremely irregular arrangement. The boundaries between bowl-shaped defects on the erosion surface and neighboring structures are unclear. The erosive areas may show granular protrusions and irregular thickened capillaries. Histopathological analysis indicates that gastric mucosal lesions that have these subtle structural features are closely related to dysplasia or even early gastric cancer. Tajiri et al[9] observed the lesions of depressed early gastric cancer and divided the patterns of microvessels on the lesion surface into six types. They found that microvessels with branches of different calibers, spiral microvessels, and microvessels of different calibers were more commonly seen in undifferentiated carcinoma. Liu et al[10] applied magnifying endoscopy to the diagnosis of gastric mucosal erosions and found that there was an indirect or direct causal relationship between gastric mucosal erosions and gastric cancer. The presence of mucosal protrusions on the erosion surface is an endoscopic feature of malignant erosions, and this is initially caused by the replacement of cancerous tissue defects by regenerative non-cancerous mucosa. As time goes on, regenerative mucosa can be replaced by cancerous tissue to form pink mucosal protrusions[11]. In our study, about 80.6% of cases of type E mucosa showed intestinal metaplasia. Under a magnifying endoscope, type F mucosa usually contains no bowl-shaped defects on the erosion surface, which are commonly found in types C-E mucosa. However, the erosion surface has become rough, and the mucosa shows granular hyperplasia or irregular changes. The boundaries between the erosion surface and surrounding structures are unclear, and gastric pits in the periphery of the lesion are sparse, irregularly arranged type E pits. Type F mucosa often suggests varying degrees of dysplasia, which has a coincidence rate of 86.7% compared with pathological results. In addition, some cases of type F mucosa that is proved to have dysplasia by histopathology show varying degrees of capillary dysplasia, which manifests as the presence of irregular, thick, disordered capillaries on the erosive mucosal surface. This characteristic change is similar to tumor vascular change described by Otsuka et al[12] and Tajiri et al[9] in early gastric cancer. Thus, the presence of types E and F mucosa can be regarded as a subtle structural feature of gastric precancerous lesions. In this study, comparison of the findings of magnifying chromoendoscopy and histopathological analysis results indicates that the presence of types E and F mucosa suggests the emergence of intestinal metaplasia and dysplasia.
The p53 gene is a tumor suppressor gene that is located on the short arm of human chromosome 17. It encodes a 53-kDa nuclear phosphoprotein that has transcription factor activity and plays an important role in the control of the cell cycle and apoptosis[13]. p53 mediates G1 arrest in response to DNA damage for DNA excision and repair to maintain the stability of the genome[14]. Wild-type p53 acts as not only a transcriptional activator but also a transcription suppressor[15]. Mutant-type p53 (mt-p53) can interfere with intracellular growth factor signaling to promote cell proliferation, suppress apoptosis, and eventually lead to cell transformation and tumor development and progression. In addition, mt-p53 can confer cell resistance to radiotherapy and chemotherapy by inhibiting apoptosis. Therefore, the biological behavior of tumors expressing mt-p53 is worse than that of tumors not expressing mt-p53[16].
Wild-type p53 induces cell differentiation. After transformed cells or cancer cells that do not express wild-type p53 were transfected with wild-type p53, the malignant phenotypes of cells were suppressed, cell growth and division were inhibited, cells were arrested at G1 phase, and in vivo tumorigenicity was decreased after inoculation into nude mice. On the other hand, introduction of p53 antisense RNA into the colon cancer cell line SW1116 significantly decreased proliferation rate and blocked cells in G0/1 phase, as revealed by flow cytometry[17].
Wild-type p53 induces apoptosis. After myeloid leukemia cell lines that do not express wild-type p53 were transfected with wild-type p53, typical apoptosis occurred, and cells lost viability. Treatment of tumors with radiation or anticancer agents not only induces DNA damage, blocks DNA metabolism, and leads to necrosis, but also induces apoptosis. The expression of transfected wild-type p53 gene can increase anticancer treatment-induced apoptosis. In contrast, tumor cells transfected with the mt-p53 gene or tumor cells not expressing wild-type p53 gene are resistant to anticancer treatment-induced apoptosis. A study has proved that p53 induces apoptosis via the mitochondrial pathway in activated thymocytes[18].
The expression of p53 protein is detectable in almost all types of somatic cells. In normal cells, wild-type p53 protein has a half-life of about 20-30 min and shows little accumulation. Finlay et al[19] found that, in actively growing tumor cells, mt-p53 protein, but not wild-type p53 protein, can form a complex with heat shock protein 70. This complex extends the half-life of mt-p53 protein, which significantly increases the accumulation of mt-p53 protein in the nucleus[20]. The p53 gene is an important tumor suppressor gene, and many human tumors, such as breast cancer, lung cancer, colorectal cancer, and gastric cancer, carry various p53 gene mutations. High expression of the mt-p53 gene is often detectable in many types of tumors and transformed cells. This study found that the expression of p53 protein in precancerous lesions (intestinal metaplasia and dysplasia) was higher than that in chronic gastritis.
PCNA was first discovered by Miyachi et al[21] in 1978 in the sera of patients with systemic lupus erythematosus. It was named as such because it is only expressed in normal proliferating cells and tumor cells. PCNA is a 36-kDa nuclear protein that is the auxiliary protein of DNA polymerase delta. Two types of nuclear PCNA exist, soluble and insoluble. Soluble PCNA is expressed in various phases of the cell cycle, and its quantity does not change significantly in the process of DNA synthesis. It is susceptible to detergent extraction and destruction by methanol. Insoluble PCNA is more stable and insusceptible to detergent elution and destruction by methanol. No obvious expression of soluble PCNA is detected in G0-Gl phase. However, its expression shows a significant increase in the late Gl phase, peaks in S phase, and decreases significantly in G2-M phase. Since the expression of soluble PCNA correlates well with DNA synthesis, it can be used as a marker for evaluation of cell proliferation. Given that cancer cells have a strong proliferative activity and that PCNA can be used as a cell proliferation marker, many domestic and foreign studies have investigated the relationship between PCNA expression and tumor development, grading, staging, radiation sensitivity, prognosis, recurrence and metastasis, death causes, and tumor markers in a variety of tumor types, and drawn many conclusions, although some of them are still controversial among different studies. In addition, some conclusions were drawn from analyses of one or several tumor types, and it remains to be studied whether they are applicable to all tumor types.
PCNA, as the auxiliary protein of DNA polymerase δ, plays an important role in the regulation of DNA replication and is closely related to the proliferative state of cells. Wu et al[22] examined the expression of PCNA in gastric cancer and precancerous lesions and found that the positive rate of PCNA in the cancer group was significantly higher than that in the control group (80.77% vs 55.36%, P < 0.01), suggesting that high PCNA expression correlates positively with cancerous transformation. These authors therefore believe that cell proliferation activity significantly increases in gastric dysplasia and intestinal metaplasia. In a study of 133 patients with early gastric cancer, Noda et al[23] found that high expression of PCNA was significantly associated with a higher rate of lymph node metastasis and a lower 5-year survival rate, indicating that PCNA expression correlates with the degree of tumor differentiation and distant metastasis. PCNA expression was significantly higher in poorly differentiated gastric cancer or gastric cancer with distant metastasis than in well differentiated gastric cancer or gastric cancer without distant metastasis. In this study, we found that PCNA expression showed an upward trend from types A to F pits. The number of cases that showed strong PCNA positivity increased significantly with the increase in the severity of lesions.
In conclusion, this study demonstrates that, compared with ordinary endoscopy, magnifying chromoendoscopy can provide more detailed information about fine mucosal morphology and has a significant advantage in the diagnosis of minute lesions. Magnifying chromoendoscopy in combination with p53 and PCNA detection is particularly helpful for the diagnosis of gastric precancerous lesions. The presence of types E and F pits, in which p53 and PCNA are highly expressed, is highly suggestive of the occurrence of early cancer, and patients developing these changes should be closely followed. If necessary, surgery or endoscopy can be considered.
Early diagnosis and treatment are important for improving the prognosis of gastric cancer. However, the widespread use of gastroscopy does not significantly improve the detection rate of early gastric cancer. Worldwide efforts are being made to improve the sensitivity of endoscopy in the detection of early cancer and precancerous lesions.
This study assessed the diagnostic value of magnifying chromoendoscopy combined with detection of proliferating cell nuclear antigen (PCNA) and p53 in the detection of gastric precancerous lesions.
Compared with ordinary endoscopy, magnifying chromoendoscopy can provide more detailed information about fine mucosal morphology and has a significant advantage in the diagnosis of minute lesions.
Magnifying chromoendoscopy is better than ordinary endoscopy; it can provide more information about mucosal morphology and has a significant advantage in the diagnosis of minute lesions. Magnifying chromoendoscopy in combination with p53 and PCNA detection is particularly helpful for the diagnosis of gastric precancerous lesions.
An interesting manuscript assessing the diagnostic value of magnifying chromoendoscopy combined with detection of PCNA and p53 in gastric precancerous lesions to increase the detection rate of early gastric cancer.
P- Reviewers Pereiralima JG, Benz C S- Editor Gou SX L- Editor Logan S E- Editor Xiong L
| 1. | Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics, 1997. CA Cancer J Clin. 1997;47:5-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1494] [Cited by in F6Publishing: 1405] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 2. | Endo M, Kawano T. Detection and classification of early squamous cell esophageal cancer. Dis Esophagus. 1997;10:155-158. [PubMed] [Cited in This Article: ] |
| 3. | Hosokawa O, Tsuda S, Kidani E, Watanabe K, Tanigawa Y, Shirasaki S, Hayashi H, Hinoshita T. Diagnosis of gastric cancer up to three years after negative upper gastrointestinal endoscopy. Endoscopy. 1998;30:669-674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 105] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Yang JM, Chen L, Fan YL, Li XH, Yu X, Fang DC. Endoscopic patterns of gastric mucosa and its clinicopathological significance. World J Gastroenterol. 2003;9:2552-2556. [PubMed] [Cited in This Article: ] |
| 5. | Chen X, Cen R, Xu FX, Xia J, Luo C, Cheng FL. Magnifying endoscopic classification of gastric pits and their clinicopathologic analysis. Zhongguo Neijing Zazhi. 2002;8:37-41. [Cited in This Article: ] |
| 6. | Huang YH, Zhou LY, Lin SR, Jin Z, Liu JJ, Ding SG, Xia ZW, Duan LP, Chang H. Magnifying endoscopic classification of gastric pits and their clinicopathological significance. Zhongguo Neijing Zazhi. 2004;10:14-17. [Cited in This Article: ] |
| 7. | Wang AY, Ye CM, Lin SR. Changes in gastric pits in intestinal metaplasia of gastric mucosa. Zhonghua Neike Zazhi. 1997;36:315-316. [Cited in This Article: ] |
| 8. | Endo T, Awakawa T, Takahashi H, Arimura Y, Itoh F, Yamashita K, Sasaki S, Yamamoto H, Tang X, Imai K. Classification of Barrett’s epithelium by magnifying endoscopy. Gastrointest Endosc. 2002;55:641-647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 149] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Tajiri H, Doi T, Endo H, Nishina T, Terao T, Hyodo I, Matsuda K, Yagi K. Routine endoscopy using a magnifying endoscope for gastric cancer diagnosis. Endoscopy. 2002;34:772-777. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Liu BY, Yuan LL, Chen X, Wang R. Diagnosis of gastric mucosal erosion by magnifying endoscopy. Xiandai Xiaohua Ji Jieru Zhenliao. 2004;9:64-66. [Cited in This Article: ] |
| 11. | Huang YH, Zhou LY, Lin SR, Jin Z, Liu JJ, Ding SG, Xia ZW, Duan LP, Chang H. Magnifying endoscopic manifestation of gastric atrophy, intestinal metaplasia or dysplasia and its diagnostic value. Zhonghua Xiaohua Neijing Zazhi. 2005;22:231-235. [Cited in This Article: ] |
| 12. | Otsuka Y, Niwa Y, Ohmiya N, Ando N, Ohashi A, Hirooka Y, Goto H. Usefulness of magnifying endoscopy in the diagnosis of early gastric cancer. Endoscopy. 2004;36:165-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Dahiya R, Deng G, Chen KM, Chui RM, Haughney PC, Narayan P. P53 tumour-suppressor gene mutations are mainly localised on exon 7 in human primary and metastatic prostate cancer. Br J Cancer. 1996;74:264-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991;51:6304-6311. [PubMed] [Cited in This Article: ] |
| 15. | Raycroft L, Wu HY, Lozano G. Transcriptional activation by wild-type but not transforming mutants of the p53 anti-oncogene. Science. 1990;249:1049-1051. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 408] [Cited by in F6Publishing: 486] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 16. | Aas T, Børresen AL, Geisler S, Smith-Sørensen B, Johnsen H, Varhaug JE, Akslen LA, Lønning PE. Specific P53 mutations are associated with de novo resistance to doxorubicin in breast cancer patients. Nat Med. 1996;2:811-814. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 527] [Cited by in F6Publishing: 524] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 17. | Cao J, Teng L, Cai X. [Inhibition effect of p53 antisense RNA on malignant phenotype of colorectal cancer cells]. Zhonghua Zhongliu Zazhi. 1997;19:123-126. [PubMed] [Cited in This Article: ] |
| 18. | Kakeji Y, Korenaga D, Tsujitani S, Baba H, Anai H, Maehara Y, Sugimachi K. Gastric cancer with p53 overexpression has high potential for metastasising to lymph nodes. Br J Cancer. 1993;67:589-593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 110] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Finlay CA, Hinds PW, Tan TH, Eliyahu D, Oren M, Levine AJ. Activating mutations for transformation by p53 produce a gene product that forms an hsc70-p53 complex with an altered half-life. Mol Cell Biol. 1988;8:531-539. [PubMed] [Cited in This Article: ] |
| 20. | Milner J, Medcalf EA. Cotranslation of activated mutant p53 with wild type drives the wild-type p53 protein into the mutant conformation. Cell. 1991;65:765-774. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 399] [Cited by in F6Publishing: 448] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 21. | Miyachi K, Fritzler MJ, Tan EM. Autoantibody to a nuclear antigen in proliferating cells. J Immunol. 1978;121:2228-2234. [PubMed] [Cited in This Article: ] |
| 22. | Wu YQ, Wang MW, Long WD. Expression of p53, bcl-2 and PCNA in gastric precancerous lesions. Academic Journal of PLA Postgraduate Medical School. 2001;22:286. [Cited in This Article: ] |
| 23. | Noda H, Maehara Y, Irie K, Kakeji Y, Yonemura T, Sugimachi K. Increased proliferative activity caused by loss of p21(WAF1/CIP1) expression and its clinical significance in patients with early-stage gastric carcinoma. Cancer. 2002;94:2107-2112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |