Published online Jul 14, 2013. doi: 10.3748/wjg.v19.i26.4172
Revised: December 17, 2012
Accepted: January 5, 2013
Published online: July 14, 2013
Processing time: 300 Days and 8 Hours
AIM: To investigate whether single endoscopist-performed percutaneous endoscopic gastrostomy (PEG) is safe and to compare the complications of PEG with those reported in the literature.
METHODS: Patients who underwent PEG placement between June 2001 and August 2011 at the Baskent University Alanya Teaching and Research Center were evaluated retrospectively. Patients whose PEG was placed for the first time by a single endoscopist were enrolled in the study. PEG was performed using the pull method. All of the patients were evaluated for their indications for PEG, major and minor complications resulting from PEG, nutritional status, C-reactive protein (CRP) levels and the use of antibiotic treatment or antibiotic prophylaxis prior to PEG. Comorbidities, rates, time and reasons for mortality were also evaluated. The reasons for PEG removal and PEG duration were also investigated.
RESULTS: Sixty-two patients underwent the PEG procedure for the first time during this study. Eight patients who underwent PEG placement by 2 endoscopists were not enrolled in the study. A total of 54 patients were investigated. The patients’ mean age was 69.9 years. The most common indication for PEG was cerebral infarct, which occurred in approximately two-thirds of the patients. The mean albumin level was 3.04 ± 0.7 g/dL, and 76.2% of the patients’ albumin levels were below the normal values. The mean CRP level was high in 90.6% of patients prior to the procedure. Approximately two-thirds of the patients received antibiotics for either prophylaxis or treatment for infections prior to the PEG procedure. Mortality was not related to the procedure in any of the patients. Buried bumper syndrome was the only major complication, and it occurred in the third year. In such case, the PEG was removed and a new PEG tube was placed via surgery. Eight patients (15.1%) experienced minor complications, 6 (11.1%) of which were wound infections. All wound infections except one recovered with antibiotic treatment. Two patients had bleeding from the PEG site, one was resolved with primary suturing and the other with fresh frozen plasma transfusion.
CONCLUSION: The incidence of major and minor complications is in keeping with literature. This finding may be noteworthy, especially in developing countries.
- Citation: Erdogan A. Single endoscopist-performed percutaneous endoscopic gastrostomy tube placement. World J Gastroenterol 2013; 19(26): 4172-4176
- URL: https://www.wjgnet.com/1007-9327/full/v19/i26/4172.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i26.4172
Percutaneous endoscopic gastrostomy (PEG) has been used widely for the enteral feeding of patients who have a functioning gastrointestinal tract but are unable to consume adequate nutrition orally. Patients with cerebrovascular diseases, Parkinson’s disease, dementia and head injury and those suffering from head and neck cancer and upper digestive tract cancer are candidates for PEG[1,2]. A PEG tube can be placed using one of four methods: push (Sachs-Vine), pull (Ponsky), introducer (Russell) or versa (t-fastener). The pull and push techniques are preferred because they offer greater safety and efficacy[3,4]. Both minor and major complications may occur during PEG placement. Major complications associated with PEG include peritonitis, gastric perforation, esophageal perforation, gastrocolocutaneous fistula, gastric outlet obstruction, necrotizing fasciitis and buried bumper syndrome. Minor complications include pneumoperitoneum, temporary ileus, hematoma, hemorrhage, wound infection, aspiration, tube dislodgement, gastroesophageal erosion, and gastric ulcer. Other gastrointestinal problems include gas distension, nausea, emesis, constipation and diarrhea[5-7]. In general practice, a PEG is placed by two endoscopists[1,8]. The aim of this study is to evaluate whether single endoscopist-performed PEG is safe and to compare the major and minor complications of PEG with those reported in the literature.
This study was approved by the Baskent University Institutional Review Board and Ethics Committee (Project No: KA12/150) and supported by the Baskent University Research Fund. Patients who underwent PEG placement between June 2001 and August 2011 at the Baskent University Alanya Teaching and Research Center were evaluated retrospectively. Patients whose PEG was placed for the first time by a single endoscopist were enrolled in the study. For all patients, the PEG was placed using the “pull method”. All of the patients were evaluated for indications for PEG, major and minor complications of PEG, nutritional status (prealbumin and albumin levels), C-reactive protein (CRP) levels and antibiotic treatment or antibiotic prophylaxis prior to PEG placement. Comorbidities and the rates, time and reasons for mortality were also evaluated, as were the reasons for PEG removal and the duration of PEG placement. The patients’ first-degree relatives were telephoned and interviewed about the complications associated with the PEG and the patients’ outcomes.
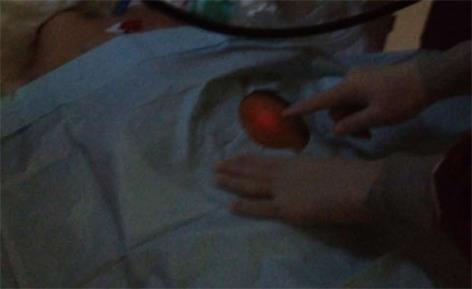
In our medical center, the standard PEG procedure was performed by single endoscopist. Before the procedure, permission for PEG placement was obtained from the patients’ first-degree relative. The procedure was performed in the intensive care unit. Lidocaine spray was administered to the throat for local anesthesia. Midazolam and/or propofol-based sedation were administered intravenously by an anesthesiologist. An upper endoscopy was performed at the beginning of the procedure to exclude severe gastric ulceration, varices and outlet obstruction. After the stomach was insufflated with air through scope, the best location for the PEG placement was determined by pressing a finger slightly against the abdominal wall. The best location was indicated by the clear indentation of the finger observed inside the stomach on the greater curvatures and the illumination of the abdominal wall (Figure 1). The nurse was then given the scope. The sterile-dressed endoscopist cleaned the abdominal wall using a povidone-iodine solution. A one-centimeter incision was made after local anesthetic was applied to the planned location. The nurse filled the patient’s stomach with air, and then the endoscopist inserted the needle of the PEG set through abdominal wall into the fully insufflated stomach. After removing the trocar, the endoscopist passed the guide wire through the needle. The nurse then caught the guide wire by the snare which was inserted through the endoscope, the endoscopist then withdrew the guide wire and the endoscope from the patient’s mouth. After the endoscopist redressed, attached the guide wire to the PEG tube and the wire was pulled out of the abdominal wall, moving the PEG tube down the esophagus. Control endoscopy was performed to optimally place the PEG tube tip. The PEG tube was turned to locate the appropriate position and was fixed with an external device, leaving a 5-mm distance between the external device and the abdominal wall. This site was cleaned with povidone-iodine solution and dressed with gauze. Enteral feeding began 24 h after the procedure and ensuring that no local wound infection was present. The patient was inspected for erythema, induration and discharge at the PEG site and was assessed using the scoring system developed by Jain et al[9] for PEG infection. The patient was also followed by the nutrition team for other complications and nutritional status until discharge. The patient’s family was asked to inform the nutrition team about possible complications.
Statistical analyses were performed using the Statistical Package for the Social Sciences software program (Version 11.0, SPSS Inc., Chicago, IL, United States).
Between June 2001 and August 2011, a total of 82 patients underwent PEG placement. Twenty patients underwent PEG replacement and were excluded from the study. Sixty-two patients underwent the PEG procedure for the first time. Eight of these procedures were performed by 2 endoscopists and were excluded from the study. A total of 54 patients were enrolled in the study. Indications were cerebral infarct in 39 patients (72.2%), cardiac arrest and cerebral ischemia in 4 patients (7.4%), dementia in 7 patients (12.9%), head trauma in 3 patients (5.6%), and cancer in 1 patient (1.9%).
Of the patients whose PEG was placed for the first time, 24 (46.3%) were women and 30 (55.6%) were men. The mean age was 69.9 years. The comorbidities accompanying the patients’ primary disease were hypertension, diabetes mellitus, cardiac arrhythmia, coronary artery disease, chronic obstructive pulmonary disease, chronic renal disease, hyperlipidemia and hydrocephaly. The mean albumin levels were 3.04 ± 0.7 g/dL, and 76.2% were below normal values. The mean CRP level was high in 90.6% of patients prior to the procedure (Table 1). In our study, 74.1% of the patients received antibiotics either for prophylaxis or for treatment for infections prior to the PEG procedure. The demographic, clinical and laboratory characteristics of the study subjects are shown in Table 1.
| Age, yr | 69.9 ± 16.3 |
| Gender, M:F | 30 (55.6):24 (46.3) |
| Comorbidities | |
| Diabetes mellitus | 19 (35.2) |
| Chronic obstructive pulmonary disease | 6 (11.1) |
| Coronary artery disease | 11 (20.4) |
| Cardiac arrhythmia | 4 (7.4) |
| Hypertension | 20 (37.0) |
| Chronic renal failure | 3 (5.6) |
| Hyperlipidemia | 2 (3.7) |
| Other1 | 4 (7.4) |
| Antimicrobial therapy prior to PEG | 40 (74.1) |
| Laboratory findings | |
| Leukocytosis (> 11 kg/mm3) | 22 (40.7) |
| CRP elevation (> 8 mg/dL) | 48 (90.6) |
| High CRP (> 80 mg/dL) | 22 (41.5) |
| Low albumin levels (< 3.5 g/dL) | 40 (76.9) |
After hospitalization, the mean time past until PEG placement was 22 ± 15.6 d. Buried bumper syndrome was the only one major complication (1.6%), and it occurred in the third year in one patient. In that case, the PEG was removed, and a new PEG tube was placed surgically. Eight patients (15.1%) experienced minor complications, 6 (11.1%) of which were wound infections and 2 of which (3.7%) were bleeding. All wound infections except for 1, which resulted in the removal of the PEG, recovered with antibiotic treatment. Two patients experienced bleeding from the PEG site; one patient was receiving anticoagulation therapy. One case resolved with primary suture, and the other resolved with fresh frozen plasma transfusion.
The first-degree relatives of all of the patients were interviewed by phone. The family members of 6 of the 54 PEG patients could not be reached by telephone, so we do not have long-term follow-up results for these patients. In our study, 1 mo survival was 85.4%, and three-month survival was 41.7%. Twenty-nine patients died during follow-up. The PEG indications for the patients who died were as follows: 14 had cerebral infarct, 3 had head trauma, 2 had cardiac arrest and cerebral ischemia and 1 had cancer. Mortality was not related to PEG placement in any of the patients and mainly depended on the underlying medical problems. The PEG tube was withdrawn in seven patients after they regained swallowing function and in one patient with an uncontrolled local wound infection. As of this writing, eleven patients live with the PEG tube, and 6 of them underwent PEG replacement during follow-up. To date, their relatives have not mentioned any problem related to the PEG in follow-up telephone interviews.
Although PEG is usually a safe procedure, certain complications can occur that may cause mortality, especially in patients with comorbidities. In our study, no mortality was associated with the PEG procedure. Buried bumper syndrome was the only major complication, and it occurred in only one patient (1.9%) in the third year of PEG placement. Minor complications occurred in 15.1% of patients, and most of these complications were wound infections.
Survival is an important endpoint in PEG studies. One-month survival is approximately 80% to 90% in most reports[10-12]. Similar to our study, the most frequent indication for PEG insertion was a neurological condition, and several studies reported that stroke was the most common indication[12-14]. In our study, one-month survival was 85.4%, and three-month survival was 41.7%. Buried bumper syndrome is an uncommon but severe complication of the procedure. It usually occurs after four months of PEG placement; however, it has also been reported to occur as late as 7 years after placement[15-17]. Rino et al[5] reported this complication as early as 5 d after the procedure. Finocchiaro et al[10] reported that one hundred twenty-eight patients were followed long-term for more than 31 d; major complications occurred in 3% of the patients, 2 of whom had buried bumper syndrome. Other major complications included 1 case of aspiration pneumonia and 1 case of subcutaneous abscess. In our study, buried bumper syndrome was the only observed major complication, and it occurred 3 years after the procedure. In the patient with buried bumper syndrome, the PEG tube was successfully surgically removed, and a new PEG tube was placed via the same procedure.
As in our study, the most common complication of PEG was infection, which sometimes results in the removal of the PEG tube[18,19]. In a prospective study in which antibiotic prophylaxis was not given, the rate of peristomal infection was 33.6%[7]. Another study reported wound infections rates of up to 18%, and antibiotic prophylaxis was shown to reduce the rate to nearly 3%[19]. In a prospective, randomized, double-blind, placebo-controlled study by Jain et al[9], antibiotic prophylaxis with cefazolin was associated with decreased local PEG site infection. In a meta-analysis by Jafri et al[20], antibiotic prophylaxis before the PEG procedure was effective in reducing postprocedure local infection rates. In our study, 74.1% of the patients received antibiotics for either prophylaxis or the treatment of infections prior to the PEG procedure. The local wound infection rate was 11.1%, which is comparable to the rates reported by other studies in the literature. Only one patient developed a PEG site infection that did not resolve with antibiotic therapy; in this case, the PEG was removed. The other minor complication in our study was bleeding from the PEG puncture site, which occurred in two patients. One patient was treated with fresh frozen plasma, and the other was treated via primary suture of the abdominal wall vessel. Bleeding from the puncture site occurs as a result of a puncture of the abdominal wall vessel soon after the procedure. It can also be treated by tightening the outside apparatus of the PEG tube. Singh et al[21] reported that gastrointestinal bleeding after PEG placement occurred in 3.3% of patients, and bleeding directly attributed to PEG was noted in 0.4%.
Many factors contribute to PEG complications. The PEG tube placement team’s experience, the PEG tube size, underlying malignancy and the institution in which the PEG procedure is performed are risk factors for wound infection. Low albumin levels and high CRP levels, age over 65 years and low BMI have also been associated with increased mortality risk[7,8,22-26]. In our study, the mean age was 69.9 years. High CRP levels were found in 41.5% of the patients, and low albumin levels were found in 76.9%. Although these unfavorable parameters existed prior to the procedure, there was no evidence of mortality related to PEG.
The nonrandomized and retrospective nature of our study are its restrictions. A prospective and randomized study might better define the safety and appropriateness of the single endoscopist-performed procedure.
In conclusion, the major and minor complications of single-endoscopist PEG are consistent with those reported in the literature for PEG procedures performed by two endoscopists. This finding may be noteworthy, especially in developing countries.
Percutaneous endoscopic gastrostomy (PEG) is lifesaving for patients who cannot feed orally for certain reasons. PEG is routinely placed by two endoscopists and carried inherent complications, some of which are life-threatening. It is not known whether PEG placement performed by a single endoscopist is safe or appropriate.
The complications of PEG are significant. No study has reported the single-endoscopist PEG procedure or its related complications.
Although this study is retrospective and lacks the advantages of prospective and randomized trials, it provides important information indicating that PEG procedures can be applied by a single endoscopist, and the complications encountered are similar to those reported in other studies of PEG performed by two endoscopists.
Single endoscopist-performed PEG may be an appropriate and safe method for performing the procedure, especially in developing countries.
This is a well-written retrospective study about the PEG procedure, which is performed here by a single endoscopist. The results show that it may be safe and appropriate for a single endoscopist to perform PEG. This study may lead to prospective and randomized trials in this field.
P- Reviewers Kawai T, Lujber L S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Zuercher BF, Grosjean P, Monnier P. Percutaneous endoscopic gastrostomy in head and neck cancer patients: indications, techniques, complications and results. Eur Arch Otorhinolaryngol. 2011;268:623-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Schrag SP, Sharma R, Jaik NP, Seamon MJ, Lukaszczyk JJ, Martin ND, Hoey BA, Stawicki SP. Complications related to percutaneous endoscopic gastrostomy (PEG) tubes. A comprehensive clinical review. J Gastrointestin Liver Dis. 2007;16:407-418. [PubMed] |
| 3. | Ponsky JL, Gauderer MW. Percutaneous endoscopic gastrostomy: a nonoperative technique for feeding gastrostomy. Gastrointest Endosc. 1981;27:9-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 282] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Urban KG, Terris DJ. Percutaneous endoscopic gastrostomy by head and neck surgeons. Otolaryngol Head Neck Surg. 1997;116:489-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Rino Y, Tokunaga M, Morinaga S, Onodera S, Tomiyama I, Imada T, Takanashi Y. The buried bumper syndrome: an early complication of percutaneous endoscopic gastrostomy. Hepatogastroenterology. 2002;49:1183-1184. [PubMed] |
| 6. | Ermis F, Ozel M, Oncu K, Yazgan Y, Demirturk L, Gurbuz AK, Akyol T, Nazik H. Indications, complications and long-term follow-up of patients undergoing percutaneous endoscopic gastrostomy: A retrospective study. Wien Klin Wochenschr. 2012;124:148-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Zopf Y, Konturek P, Nuernberger A, Maiss J, Zenk J, Iro H, Hahn EG, Schwab D. Local infection after placement of percutaneous endoscopic gastrostomy tubes: a prospective study evaluating risk factors. Can J Gastroenterol. 2008;22:987-991. [PubMed] |
| 8. | Blomberg J, Lagergren P, Martin L, Mattsson F, Lagergren J. Albumin and C-reactive protein levels predict short-term mortality after percutaneous endoscopic gastrostomy in a prospective cohort study. Gastrointest Endosc. 2011;73:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Jain NK, Larson DE, Schroeder KW, Burton DD, Cannon KP, Thompson RL, DiMagno EP. Antibiotic prophylaxis for percutaneous endoscopic gastrostomy. A prospective, randomized, double-blind clinical trial. Ann Intern Med. 1987;107:824-828. [PubMed] |
| 10. | Finocchiaro C, Galletti R, Rovera G, Ferrari A, Todros L, Vuolo A, Balzola F. Percutaneous endoscopic gastrostomy: a long-term follow-up. Nutrition. 1997;13:520-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 94] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Bourdel-Marchasson I, Dumas F, Pinganaud G, Emeriau JP, Decamps A. Audit of percutaneous endoscopic gastrostomy in long-term enteral feeding in a nursing home. Int J Qual Health Care. 1997;9:297-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Nicholson FB, Korman MG, Richardson MA. Percutaneous endoscopic gastrostomy: a review of indications, complications and outcome. J Gastroenterol Hepatol. 2000;15:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 147] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Suzuki Y, Tamez S, Murakami A, Taira A, Mizuhara A, Horiuchi A, Mihara C, Ako E, Muramatsu H, Okano H. Survival of geriatric patients after percutaneous endoscopic gastrostomy in Japan. World J Gastroenterol. 2010;16:5084-5091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Gomes CA, Lustosa SA, Matos D, Andriolo RB, Waisberg DR, Waisberg J. Percutaneous endoscopic gastrostomy versus nasogastric tube feeding for adults with swallowing disturbances. Cochrane Database Syst Rev. 2012;3:CD008096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Erdil A, Genç H, Uygun A, Ilica AT, Dağalp K. The buried bumper syndrome: the usefulness of retrieval PEG tubes in its management. Turk J Gastroenterol. 2008;19:45-48. [PubMed] |
| 16. | Vargo JJ, Ponsky JL. Percutaneous endoscopic gastrostomy: clinical aplication. MedGenMed. 2000;2. |
| 17. | Gençosmanoğlu R, Koç D, Tözün N. The buried bumper syndrome: migration of internal bumper of percutaneous endoscopic gastrostomy tube into the abdominal wall. J Gastroenterol. 2003;38:1077-1080. [PubMed] |
| 18. | Richter-Schrag HJ, Richter S, Ruthmann O, Olschewski M, Hopt UT, Fischer A. Risk factors and complications following percutaneous endoscopic gastrostomy: a case series of 1041 patients. Can J Gastroenterol. 2011;25:201-206. [PubMed] |
| 19. | Ahmad I, Mouncher A, Abdoolah A, Stenson R, Wright J, Daniels A, Tillett J, Hawthorne AB, Thomas G. Antibiotic prophylaxis for percutaneous endoscopic gastrostomy--a prospective, randomised, double-blind trial. Aliment Pharmacol Ther. 2003;18:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Jafri NS, Mahid SS, Minor KS, Idstein SR, Hornung CA, Galandiuk S. Meta-analysis: antibiotic prophylaxis to prevent peristomal infection following percutaneous endoscopic gastrostomy. Aliment Pharmacol Ther. 2007;25:647-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | Singh D, Laya AS, Vaidya OU, Ahmed SA, Bonham AJ, Clarkston WK. Risk of bleeding after percutaneous endoscopic gastrostomy (PEG). Dig Dis Sci. 2012;57:973-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Tominaga N, Shimoda R, Iwakiri R, Tsuruoka N, Sakata Y, Hara H, Hayashi S, Morita S, Hamasaki Y, Matsushima T. Low serum albumin level is risk factor for patients with percutaneous endoscopic gastrostomy. Intern Med. 2010;49:2283-2288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Friedenberg F, Jensen G, Gujral N, Braitman LE, Levine GM. Serum albumin is predictive of 30-day survival after percutaneous endoscopic gastrostomy. JPEN J Parenter Enteral Nutr. 1997;21:72-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Lang A, Bardan E, Chowers Y, Sakhnini E, Fidder HH, Bar-Meir S, Avidan B. Risk factors for mortality in patients undergoing percutaneous endoscopic gastrostomy. Endoscopy. 2004;36:522-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Yokohama S, Aoshima M. [Risk factors of early mortality after percutaneous endoscopic gastrostomy: a retrospective study]. Nihon Shokakibyo Gakkai Zasshi. 2009;106:1313-1320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Richards DM, Tanikella R, Arora G, Guha S, Dekovich AA. Percutaneous endoscopic gastrostomy in cancer patients: predictors of 30-day complications, 30-day mortality, and overall mortality. Dig Dis Sci. 2013;58:768-776. [PubMed] |