Published online Apr 14, 2013. doi: 10.3748/wjg.v19.i14.2217
Revised: November 27, 2012
Accepted: December 20, 2012
Published online: April 14, 2013
Processing time: 158 Days and 13.7 Hours
AIM: To investigate the evolution of disease phenotype in adult and pediatric onset Crohn’s disease (CD) populations, diagnosed between 1977 and 2008.
METHODS: Data of 506 incident CD patients were analyzed (age at diagnosis: 28.5 years, interquartile range: 22-38 years). Both in- and outpatient records were collected prospectively with a complete clinical follow-up and comprehensively reviewed in the population-based Veszprem province database, which included incident patients diagnosed between January 1, 1977 and December 31, 2008 in adult and pediatric onset CD populations. Disease phenotype according to the Montreal classification and long-term disease course was analysed according to the age at onset in time-dependent univariate and multivariate analysis.
RESULTS: Among this population-based cohort, seventy-four (12.8%) pediatric-onset CD patients were identified (diagnosed ≤ 17 years of age). There was no significant difference in the distribution of disease behavior between pediatric (B1: 62%, B2: 15%, B3: 23%) and adult-onset CD patients (B1: 56%, B2: 21%, B3: 23%) at diagnosis, or during follow-up. Overall, the probability of developing complicated disease behaviour was 49.7% and 61.3% in the pediatric and 55.1% and 62.4% in the adult onset patients after 5- and 10-years of follow-up. Similarly, time to change in disease behaviour from non stricturing, non penetrating (B1) to complicated, stricturing or penetrating (B2/B3) disease was not significantly different between pediatric and adult onset CD in a Kaplan-Meier analysis. Calendar year of diagnosis (P = 0.04), ileal location (P < 0.001), perianal disease (P < 0.001), smoking (P = 0.038) and need for steroids (P < 0.001) were associated with presence of, or progression to, complicated disease behavior at diagnosis and during follow-up. A change in disease location was observed in 8.9% of patients and it was associated with smoking status (P = 0.01), but not with age at diagnosis.
CONCLUSION: Long-term evolution of disease behavior was not different in pediatric- and adult-onset CD patients in this population-based cohort but was associated to location, perianal disease and smoking status.
- Citation: Lovasz BD, Lakatos L, Horvath A, Szita I, Pandur T, Mandel M, Vegh Z, Golovics PA, Mester G, Balogh M, Molnar C, Komaromi E, Kiss LS, Lakatos PL. Evolution of disease phenotype in adult and pediatric onset Crohn’s disease in a population-based cohort. World J Gastroenterol 2013; 19(14): 2217-2226
- URL: https://www.wjgnet.com/1007-9327/full/v19/i14/2217.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i14.2217
Inflammatory bowel disease (IBD) is multifactorial: both genetic and environmental risk factors (e.g., smoking, or appendectomy) contribute to its pathogenesis[1]. During the past two decades, the incidence pattern of IBD has changed significantly[2], showing both common and distinct characteristics. The phenotypic classification of Crohn’s disease (CD) plays an important role in patient management, and may help predict the clinical course in CD patients[3]. In 2005, the Montreal revision of the Vienna classification system was introduced[4]. Although the broad categories for CD classification remained the same, changes were made within each category. Upper gastrointestinal (GI) disease is now classified independently of, or alongside, disease at more distal locations. Finally, perianal disease, which occurs independently of small bowel fistulae, is no longer classified as penetrating disease. Instead, a perianal modifier has been introduced, which may coexist with any disease behavior.
Using the Vienna classification system, it has been shown in clinical cohorts that there can be a significant change in disease behavior over time, whereas disease location remains relatively stable[3,5]. In a landmark paper by Cosnes et al[6], up to 70% of CD patients developed either penetrating or stricturing disease. Louis et al[5] reported similar results in a Belgian study, in which 45.9% of patients had a change in disease behavior (P < 0.0001) during 10 years of follow-up, especially from non-stricturing, non-penetrating disease to either stricturing (27.1%; P < 0.0001) or penetrating (29.4%; P < 0.0001) forms. Age at diagnosis (before or after age 40) had no influence on either disease location or behavior. In contrast, disease location remained relatively stable during follow-up, with only 15.9% of patients exhibiting a change in disease location during the first 10 years. In addition, the probability of change in disease behavior in patients with initially non-stricturing, non-penetrating disease was 30.8% over nine years in a more recent Hungarian study[7], in which data were obtained from referral centers.
More recently, authors from New Zealand[3] showed in a population-based cohort study, that although > 70% percent of CD patients had inflammatory disease at diagnosis, 23% and 40% of patients with initial inflammatory disease progressed to complicated disease phenotypes after five and ten years of follow-up, respectively. This was not associated with age at onset. In contrast, disease location remained stable in 91% of patients with CD. Of note however, the median follow-up of CD patients was only 6.5 years. Similarly, in the IBSEN cohort, 36%, 49% and 53% of CD patients diagnosed between 1990 and 1994 initially had or developed either stricturing or penetrating complications[8]. In addition, recent data suggest a change in the natural history of CD as shown by decreasing surgical rates[9].
According to the available literature, pediatric onset CD runs a more aggressive course, including more extensive disease location, more upper GI involvement, growth failure, more active disease, and need for more aggressive medical therapy, in predominantly referral-center studies[10-12]. However, data so far have been partly contradictory, and pediatric disease behavior seems to parallel that of adults[13]. A Scottish study simultaneously compared disease behavior and location in pediatric and adult onset IBD patients[14]. In childhood-onset patients, there was a clear difference in disease location at onset and after five years; with less ileum- and colon-only location among pediatric-onset patients, but more ileocolonic and upper gastrointestinal involvement (P < 0.001 for each). In addition, disease behavior after five years did not differ between the two groups. In contrast, disease phenotype was associated with location. However, the evolution of disease phenotype was not studied.
Because only limited data are available on the evolution of disease phenotype in patients with a pediatric- and adult-onset CD in from a single population-based cohort over a long-term follow-up, the aim of this study was to analyze the evolution of disease behavior and location in a population-based Veszprem province database according to the age-group at diagnosis, which included incident adult- and pediatric-onset CD populations diagnosed between January 1, 1977 and December 31, 2008.
A well-characterized Hungarian cohort of 1420 incident cases of inflammatory bowel disease diagnosed between January 1, 1977 and December 31, 2008 were included. In total, 506 CD patients [CD, male: female: 251:255, age at diagnosis: 28.5 years, interquartile range (IQR): 22-38 years] were diagnosed during the inclusion period. Patients were followed until December 31, 2009 or death. All patients had at least one year of follow-up data available. Patients with indeterminate colitis at diagnosis were excluded from the analysis. Patient clinical data is summarized in Table 1. The ratio of urban-to-rural residence was also relatively stable (55% urban).
| CD (n = 506) | |
| Male/female | 251/255 |
| Age at presentation (yr)1 | 28.5 (22-38) |
| Follow-up (yr)1 | 13.5 (6-19.5) |
| Familial IBD | 12.90% |
| Location at diagnosis | |
| L1 | 32.80% |
| L2 | 35.90% |
| L3 | 30.60% |
| L4 only | 0.70% |
| L4 | 4.80% |
| Behavior at diagnosis | |
| B1 | 56.90% |
| B2 | 19.80% |
| B3 | 23.30% |
| Frequent relapse | 13.10% |
| Perianal disease | 25.50% |
| Arthritis | 26.70% |
| PSC | 1.80% |
| Ocular | 4.70% |
| Cutaneous | 9.30% |
| Steroid use | 68.60% |
| Azathioprine use | 45.80% |
| Biological use | 10.70% |
| Resection/re-operation | 41.3%/28.2% |
Data collected from 7 general hospitals and gastroenterology outpatient units (Internal Medicine Departments, Surgery Departments, Paediatric Departments and Outpatient Units) from Veszprem County (Veszprem, Papa, Tapolca, Ajka, Varpalota, Zirc). A more detailed description of the data collection and case assessment methods used, as well as the geographical and socioeconomic background of the province and the Veszprem Province IBD Group was published in previous epidemiological studies by this group[15].
The majority of patients (94% of CD and 71% of ulcerative colitis patients) were monitored at the Csolnoky F Province Hospital in Veszprem. This hospital also serves as a secondary referral center for IBD patients in the province. Data collection was prospective since 1985; prior to that, only in Veszprem were data collected prospectively. In other sites throughout the province, data for this period (1977-1985) were collected retrospectively in 1985. Both in- and outpatients permanently residing in the area were included in the study. Diagnoses (based on hospitalization records, outpatient visits, endoscopic, radiological, and histological evidence) generated in each hospital and outpatient unit were reviewed thoroughly, using the Lennard-Jones[16] or the Porto criteria[17], as appropriate. At the Veszprem pediatric IBD clinic, all probable cases of IBD are evaluated in a single unit by a pediatric gastroenterologist with experience in the diagnosis and treatment of IBD together with adult gastroenterologists. In addition, all endoscopies for pediatric patients are performed and all follow-up is conducted by two expert adult gastroenterologists, and pediatric cases were followed together by pediatric and adult gastroenterologists. According to the Montreal classification, an age at diagnosis < 17 years was defined as pediatric onset.
Age, age at onset, the presence of familial IBD, presence of extraintestinal manifestations (EIM) including: arthritis, conjunctivitis, uveitis, episcleritis, erythema nodosum, pyoderma gangrenosum, primary sclerosing cholangitis (PSC), and the frequency of flare-ups (frequent flare-up: > 1/year[18]) were registered. Disease phenotype (age at onset, duration, location, and behavior) was determined according to the Montreal classification[4] (based on: age at onset, location, and behavior, with perianal and upper GI disease as additional modifiers). Non-inflammatory behavior was defined as either stricturing or penetrating disease. Perianal disease and behavior change (from B1 to B2 or B3) or location during follow-up was also registered. Every significant flare or new symptom was meticulously investigated by gastroenterology specialists. Morphological investigations included proctosigmoidoscopy, colonoscopy, computed tomography (CT) scan, small-bowel ultrasound and small bowel X-ray. Patients in clinical remission had regular follow-up visits including laboratory and imaging studies (annual abdominal ultrasound). Endoscopy and CT-scans were only occasionally performed in patients in clinical remission. Of note, upper GI symptoms were carefully evaluated. Only indisputable manifestations were classified as upper GI involvement (e.g., stenosis, ulcers), but not small erosions, or even simple gastric or duodenal ulcers, the later occurring shortly after the start of high dose systemic steroid therapy. Upper GI endoscopy was performed regularly at the time on the diagnosis of CD only in the last ten years, earlier only in case of gastroesophageal symptoms.
Medical therapy was thoroughly registered (e.g., steroid, immunosuppressive, or biological use, azathioprine intolerance as defined by the European Crohn’s and Colitis Organization, Consensus Report 28), need for surgery or reoperation (resections in CD), development of colorectal and small bowel adenocarcinoma, other malignancies, and smoking habits, were investigated by reviewing medical records during follow-up and by the completion of a questionnaire. Only patients with a confirmed diagnosis for more than one year were enrolled.
In addition, due to Hungarian health authority regulations, a follow-up visit is obligatory for IBD patients at a specialized gastroenterology center every six months. Otherwise, under the conditions of the Hungarian national health insurance system, patients forfeit their right to ongoing subsidized therapy. Consequently, the relationship between IBD patients and specialists is a close one.
The study was approved by the Semmelweis University Regional and Institutional Committee of Science and Research Ethics and the Csolnoky F Province Hospital Institutional Committee of Science and Research Ethics.
Variables were tested for normality by Shapiro Wilk’s W-test. The distribution of disease behavior at different time points and between subgroups of CD patients was compared by χ2-test with Yates correction. Odds ratios (OR) were calculated. Kaplan-Meier survival curves were plotted for analysis with LogRank and Breslow tests to determine probability of disease behavior change in patients with inflammatory (B1) behavior at diagnosis. Additionally, Cox-regression analysis using the enter method was used to assess the association between categorical clinical variables and time to disease behavior or location change. Variables with a P value < 0.2 in univariate analysis were included in the multivariate testing results for continuous variables are expressed as median (IQR) unless otherwise stated. Peter Laszlo Lakatos performed all statistical analysis. For statistical analysis, SPSS® 15.0 (SPSS Inc., Chicago, IL) was used. A P value of < 0.05 was considered significant.
Five hundred six residents of the Veszprem province were diagnosed with CD during the 32-year period from 1977 to 2008. The clinical characteristics of these patients are shown in Table 1. Sixty-five (12.8%) CD patients were diagnosed < 17 years of age. Follow-up information was collected up to December 31 2009, equaling 5758 patient-years of follow-up.
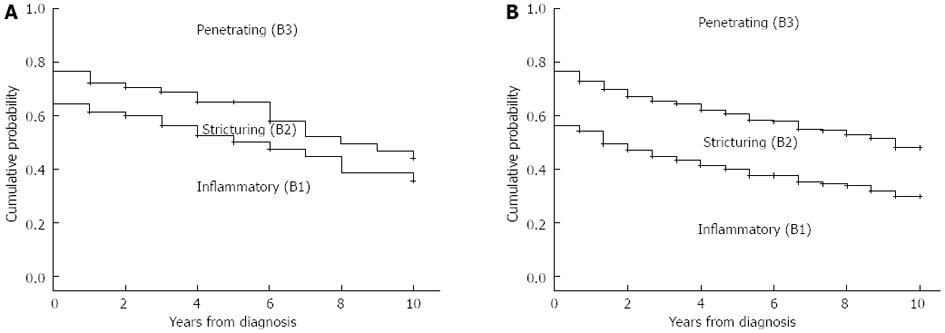
There was no significant difference in the distribution of disease behavior between pediatric (B1: 62%, B2: 15%, and B3: 23%) and adult onset CD patients (B1: 56%, B2: 21%, and B3: 23%) at diagnosis (P = NS). In addition, the distribution of disease behavior after 1, 3, 5, 7, 10 and 15 years and the probability of developing penetrating or complicated (stenosing/penetrating) disease behavior during follow-up did not significantly differ in patients with pediatric and adult onset disease by χ2 and Kaplan-Meier analysis (Figure 1, PLogRank = NS, PBreslow = NS) Because the length of follow-up differed between the groups, statistical analysis was not performed using final disease behavior data.
Similarly, the probability and time to change in disease behavior from B1 to B2/B3 disease was not significantly different between pediatric- and adult-onset CD in a Kaplan-Meier analysis (Figure 2). The probability of complicated disease behavior for patients who initially exhibited inflammatory disease behavior was 7.6%, 27.5%, and 42.0% in the pediatric and 12.1%, 26.4%, and 37.5% in the adult-onset patients after 1, 5, and 10 years of follow-up (PLogRank = NS, PBreslow = NS).
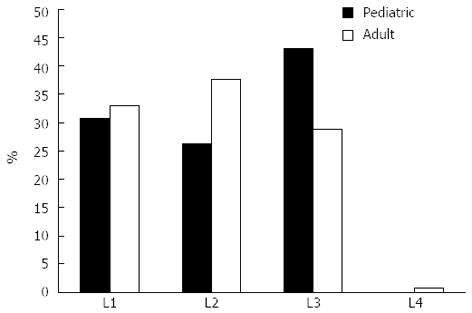
In contrast, the distribution of disease location at diagnosis was different between pediatric- and adult-onset CD patients (L3 pediatric-onset: 41.3%, vs adult-onset: 28.8% P = 0.05, Figure 3). A change in disease location was observed 8.9% of the CD patients. The probability of change in disease location was 5.2%, 8.9%, and 10.8% after 5, 10, and 15 years of follow-up, respectively. However, this did not differ according to age at onset (PLogRank = NS).
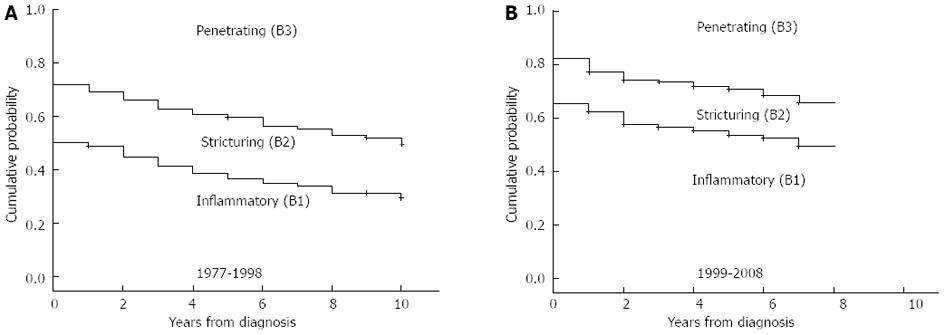
The calendar year of diagnosis and location were associated with presence of or progress to complicated disease behavior at diagnosis and during follow-up. There was a significant difference in the distribution of disease behavior in patients diagnosed from 1977 to 1998 (n = 273, B1: 50%, B2: 22%, and B3: 28%) and from 1999 to 2008 (B1: 65%, B2: 17% and B3: 18%) at diagnosis (P = 0.003) and after one, three, and five years of follow-up (P1-year = 0.007, P3-years = 0.002, P5-years < 0.001 by χ2 analysis, and in the probability of developing penetrating or complicated (stenosing/penetrating) disease behavior during follow-up in a Kaplan-Meier analysis [PLogRank < 0.001, PBreslow < 0.001 (Figure 4)]. The probabilities of penetrating or complicated (stenosing/penetrating) disease behavior after three and seven years of follow-up were 37.4% and 44.8%, and 58.4% and 66.2%, in the 1977-1998 cohort, while this was 26.5% and 34.4%, and 43.6% and 50.6%, in the 1999-2008 cohort.
Trends were similar when pediatric-onset and adult-onset patients were analyzed separately. The disease behavior pattern at diagnosis did not differ significantly between the two groups diagnosed in 1977-1998 (pediatric-onset n = 33, B1: 51%, B2: 21%, and B3: 28%, adult-onset n = 240, B1: 50%, B2: 22%, and B3: 28%) and in 1999-2008 (pediatric-onset n = 32, B1: 72%, B2: 9%, and B3: 19%, adult-onset n = 201, B1: 64%, B2: 18%, and B3: 18%). The evolution of disease behavior was also similar to the full cohort (data not shown).
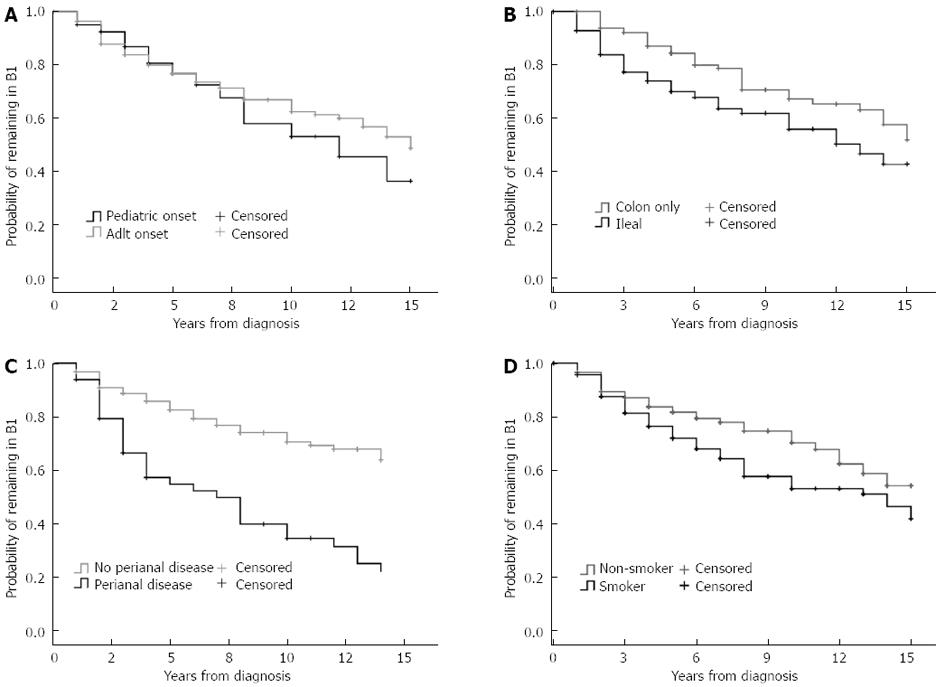
In addition, disease location and presence of perianal disease was associated with disease behavior at diagnosis (colon only B1: 73%, B2: 12%, and B3: 15%, vs ileal involvement B1: 48%, B2: 24%, and B3: 28%, P < 0.001; perianal disease absent B1: 66%, B2: 21%, and B3: 13%, perianal disease present B1: 30%, B2: 15%, and B3: 55%, P < 0.001). Probability of change in disease behavior from B1 to B2/B3 disease was significantly higher in patients with ileal involvement (PLogRank = 0.013, PBreslow = 0.002, HRL1 or L3 = 2.27, 95%CI: 1.32-3.92) and perianal disease (PLogRank < 0.001, PBreslow < 0.001, HRperianal = 2.98, 95%CI: 2.21-4.03) (Figure 2B-D). Similarly, need for steroids, either at diagnosis or during follow-up, was associated with an increased risk of disease progression (PLogRank < 0.001, PBreslow < 0.001, HR = 3.66, 95%CI: 1.67-8.04), but not early azathioprine exposure. The same trend was observed for smoking (PLogRank = 0.038, PBreslow = 0.051, HRsmoking = 1.482, 95%CI: 0.96-2.37).
In contrast, calendar year of diagnosis was associated with the progression to non-inflammatory disease behavior (PLogRank = 0.04, PBreslow = 0.04, HRafter 1998 = 0.73, 95%CI: 0.55-0.97) in patients with initially inflammatory disease. The probability of progression to complicated disease behavior after five and seven years was 15.1% and 21.8% in patients diagnosed after 1998 while this was 27.4% and 33.3% in patients diagnosed between 1977 and 1998. In a multivariate Cox regression analysis, after excluding steroid exposure at any time point from the variables, the effect of location (P < 0.001; for L1 P < 0.001, HR = 2.19, 95%CI: 1.50-3.09; for L3 P = 0.01, HR = 1.59, 95%CI: 1.12-2.28), perianal disease (P < 0.001, HR = 3.11, 95%CI: 2.23-4.34) and smoking (P = 0.031, HR = 1.42, 95%CI: 1.04-1.96) remained significant.
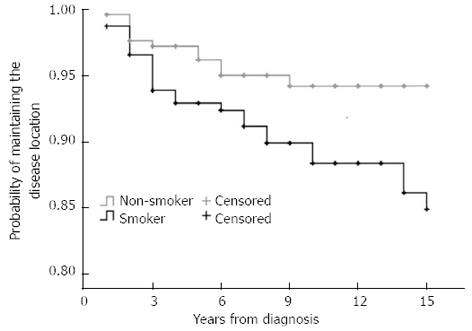
Interestingly, the probability of disease location change differed according to smoking status (PLogRank = 0.011, PBreslow = 0.03, HRsmoking = 2.35, 95%CI: 1.19-4.63, Figure 5), but not according to gender, initial disease location, behavior, presence of perianal disease or calendar year of diagnosis.
This current, population-based, prospective study reveals that evolution of disease behavior and location do not differ significantly between CD patients with adult or pediatric onset during long-term follow-up, as the change of disease phenotype is not different significantly in pediatric- and adult-onset CD, in contrast to previous, large, multicenter studies. There were no significant differences in disease behavior between pediatric- and adult-onset patients at the time of diagnosis or during follow-up. In contrast, findings presented here confirm the results of previous studies, namely, that disease location was significantly different according to the age at diagnosis. Pediatric patients presented more frequently with extensive disease. A change in disease location was relatively rare and it was associated with smoking status.
The same, progressive characteristics of CD were described by Cosnes et al[6] in a study of patients treated at a French referral center. More than 80% of patients developed complications with time. After 5 and 20 years’ disease duration, the risk for stricturing disease complications were 12% and 18% respectively, whereas 40% and 70% of patients developed penetrating complications, respectively. An association was reported, however, with disease location; the probability of complicated disease was as high as 94% after 20 years in patients with ileal disease. Results were comparable in a Belgian study[5]. In this study, 45.9% of patients had a change in disease behavior after 10 years of follow-up, especially from non-stricturing, non-penetrating disease to either stricturing (27.1%) or penetrating (29.4%) disease. The frequency of complicated disease was somewhat lower in the present population-based study. The probability of developing penetrating complications was 35.4% and 58.2% after 5 and 20 years’ disease duration, while 55.9% and 73.7% of patients diagnosed between 1977-2008 developed either penetrating or stricturing complications. Finally, 53% of patients developed stricturing or penetrating disease during a 10-year follow-up in the population-based prospective IBSEN cohort in CD patients diagnosed between 1990 and 1994[8]. Of note, however, in the study by Cosnes et al[6], classification of patients according to disease behavior was a poor predictor of disease activity during the next five years. A similar proportion of patients required immunosuppressive drugs and surgery.
According to previous data, the natural history was reported to be more severe in pediatric CD. Extensive, complicated disease phenotypes were reported to be frequent in a population-based study by Vernier-Massouille et al[19] In this study, the prevalence of B2 and B3 phenotypes increased from 25% to 44%, and from 4% to 15%, while the frequency of B1 disease decreased from 71% to 41%, respectively, from diagnosis until approximately 10 years of follow-up. In addition, according to a recent French study by Pigneur et al[10] patients with early childhood-onset onset CD often have more severe disease, increased frequency of active periods, and increased need for immunosuppressants. In contrast, in the present study, disease behavior at diagnosis and the rate of progression to complicated disease did not differ between pediatric- and adult-onset CD patients. Similarly, in a population-based cohort in New Zealand[3], age at diagnosis was not predictive of the rate of progression from inflammatory to complicated disease behavior. Until now, this was the only study that investigated the importance of age at onset according to the Montreal classification including pediatric onset patients. However, significant data were collected retrospectively and the median follow-up was 6.5 years which is half of the median follow-up of patients in the present study. In addition, > 70% of CD patients had inflammatory disease at diagnosis, with 23% and 40% of patients with initial inflammatory disease progressing to complicated disease phenotypes after five and ten years of follow-up.
Previous studies suggested that the disease location was different between pediatric and adult onset patients with more ileocolonic and upper GI disease in pediatric patients[3,6,19], in concordance with the present study. In a French population-based pediatric CD study[19] the most frequent location at diagnosis was ileocolonic disease (63%). Disease extension was observed in a surprisingly large proportion of pediatric patients (31%) during follow-up. In addition, in a population-based New-Zealand CD cohort, authors have reported an association between initial disease location and probability of disease extension. Patients with colon-only location progressed more rapidly to ileocolonic disease than those with ileal disease (P = 0.02). Of note, the rate of disease location change at 10 years in this study (9%) was in the range reported in the present study (8.9% during a median 13 years), although somewhat higher rates were reported in the study by Louis et al[5] (15.9% during 10-years). In the latter study, 20.3% of patients with an initial L1 location changed to another location, while the proportion of patients changing from L2 was 16.7%. In the present study, the probability of disease behavior change was 8.8% and 10.9% after 10 and 15 years of disease duration. The change in disease location was not different between patients with pediatric or adult onset, nor between patients with L1 and L2 disease. In contrast, a novel finding of the present study was that change in disease location was associated with smoking status (HR = 2.35, P = 0.01). The probability of a change in disease location was 5.8% and 5.8% in non-smokers, and 11.7% and 15.1% in smokers after 10 and 15 years’ disease duration.
Additional predictors of disease behavior change identified in the present study included presence of ileal involvement, perianal disease, smoking and calendar year of diagnosis, with perianal involvement being the most important predictor. The role of initial ileal involvement, extensive disease, and perianal disease as a possible predictor of non-inflammatory behavior was first suggested in a landmark study by Cosnes et al[6]. Additionally age < 40 years at diagnosis was associated with the development of penetrating complications (HR = 1.3). Similar findings were presented from the New Zealand cohort[3], where patients with ileal (L1) disease progressed most quickly to non-inflammatory disease behavior, followed by patients with upper GI (L4) or ileocolonic (L3) disease (P < 0.0001). The probability of progression to penetrating disease was similar to that of progression to stenosing disease after 10 years. Overall, the proportion of penetrating disease was highest in those with ileocolonic (27%) or ileal disease (21%) compared to patients with colon-only disease (7%, P = 0.006). Patients with perianal disease were at risk of a change in disease behavior (HR = 1.62, 95%CI: 1.28-2.05). In a subsequent population-based study from the IBSEN group[8], non-inflammatory disease behavior during follow-up was associated with initial L1 (86%) vs L2 (30%, P < 0.001) or L3 location (60%, P < 0.005). Finally, in a previous publication by our group[7], ileal disease location (HR = 2.13, P = 0.001), presence of perianal disease (HR = 3.26, P < 0.001), prior steroid use (HR = 7.46, P = 0.006), early AZA (HR = 0.46, P = 0.005) and smoking (HR = 1.79, P = 0.032) were independent predictors of disease behavior change in a referral CD cohort. Data regarding the effect of smoking are equivocal, however. A recent review[20] and previous studies have demonstrated that smoking was associated with complicated disease, penetrating intestinal complications[21], and greater likelihood of progression to complicated disease, as defined by development of strictures or fistulae, a higher relapse rate, and need for steroids and immunosuppressants[22]. In a recent study by Aldhous et al[23], the deleterious effect of smoking was only partially confirmed. Current smoking was associated with less colonic disease, however smoking habits at diagnosis were not associated with time to development of stricturing, penetrating disease, nor with perianal penetrating disease or time to first surgery. Of note, a possible neutralizing effect of immunosuppressant therapy was reported in some studies[24,25].
Conclusions were slightly different if authors assessed the factors associated with the development of disabling disease. In the paper by Loly et al[26] stricturing behavior at diagnosis (HR = 2.11, P = 0.0004) and weight loss (> 5 kg) at diagnosis (HR = 1.67, P = 0.0089) were independently associated with time to the development of severe disease in multivariate analysis. The definition of severe, non-reversible damage was, however, much more rigorous. It was defined by the presence of at least one of the following criteria: the development of complex perianal disease, any colonic resection, either two or more small-bowel resections or a single small-bowel resection measuring more than 50 cm, or the construction of a definite stoma. In a similar study by Beaugerie et al[27], with a different definition of disabling disease, initial requirement for steroid use (OR = 3.1, 95%CI: 2.2-4.4), an age below 40 years (OR = 2.1, 95%CI: 1.3-3.6), and the presence of perianal disease (OR = 1.8, 95%CI: 1.2-2.8) were associated with the development of disabling disease[27]. The positive predictive value of disabling disease in patients with two and three predictive factors of disabling disease was 0.91 and 0.93, respectively. Concordantly, in the present study, need for steroids was identified as a risk factor for progression of disease behavior (HR = 3.66, P < 0.001).
Finally, the calendar year of diagnosis was associated with disease behavior at diagnosis and the progression to non-inflammatory disease behavior (PLogRank = 0.04, PBreslow = 0.04, HR1after 1998 = 0.73, 95%CI: 0.55-0.97) in patients with initially inflammatory disease in the present study, suggesting a change in the natural history of the disease in the last decade. Trends were similar in the pediatric- and adult-onset patients. However, although azathioprine was started more frequently and earlier in the last decade[28], the change in disease behavior progression was not directly associated with the increased and earlier use of azathioprine, pointing to the fact that probably the change in the patient management is far more complex. Of note, distribution of disease location was not different in patients with a diagnosis before or after 1998. In contrast, presence of perianal disease was less prevalent in the later group (17.9% vs 31.5%, P < 0.001, OR = 0.48), suggesting and increased awareness and probably earlier diagnosis.
Authors are aware of possible limitations of the present study. The treatment and monitoring paradigm for CD patients has changed significantly over the last three decades. The majority of patients received maintenance therapy with sulfasalazine or a 5-aminosalicylic acid derivative (mesalazine or olsalazine), if tolerated, especially until the mid-1990s. Azathioprine or 6-mercaptopurine were used as maintenance therapy for steroid dependent, steroid-refractory, or fistulizing patients in selected cases, mainly after resective surgery until the late-1980s, but on a more widespread basis and earlier in the disease course only from the mid-to-late 1990s. Short-term oral corticosteroid treatment was used for clinical exacerbations, usually at initial doses of 40-60 mg of prednisone per day, which was tapered and discontinued over 2 to 3 mo. Infliximab (and later adalimumab) has been used for both induction and maintenance therapy in selected cases since the late 1990s. Similarly, small-bowel follow through was replaced by CT or MR-enterography from the 1990s. The strengths of the study include long-term prospective follow-up, the fact that leading IBD specialists were involved during the entire follow-up, and also that the evaluation and monitoring of pediatric-onset patients was managed jointly by pediatric and adult gastroenterologists using similar principles.
In conclusion, the long-term evolution of disease behavior in pediatric- and adult-onset CD patients did not differ in this population-based incident cohort. In contrast location, smoking, and need for steroids were associated with presence of, or progression to, complicated disease behavior at diagnosis and during follow-up, in concordance with previous referral and population-based studies. In addition, there was a change in the evolution of the disease behavior according to the calendar year of diagnosis. Progression to complicated disease phenotype was less likely in patients diagnosed after 1998, however this was at least partly associated with a milder disease phenotype at diagnosis including a decreased prevalence of perianal disease in the later group. A novel finding of the present study was that the change in disease location was associated with smoking status.
According to the available literature, pediatric onset Crohn’s disease (CD) runs a more aggressive course, including more extensive disease location, more upper gastrointestinal involvement, growth failure, more active disease, and need for more aggressive medical therapy, in predominantly referral-center studies.
Limited data are available on the long-term disease course in pediatric and adult patient cohorts with inflammatory bowel diseases from the same geographic area in population-based cohorts.
Some new data indicate that pediatric disease may parallel that of adults, however data so far are conflictive. The present study reports that the long-term evolution of disease behavior was not different in pediatric- and adult-onset CD patients in this prospective population-based incident cohort from Eastern Europe. Interestingly, change in disease location was associated with smoking status.
Understanding the evolution of the disease course in CD may lead to more optimized patient managment and follow-up.
Disease phenotype is categorized according to the Montreal classification and includes age at onset (A1: < 17 years, A2: 17-40 years and A3: > 40 years) location (L1: Ileal, L2: Colon, L3: Ileocolon, L4: Upper gastrointestinal) and behavior (B1: Inflammatory, B2: Stenosing, B3: Penetrating). While disease location is thought to be more stable, a change in the disease behavior is a rather frequent event.
This is a prospective, well-designed study, with a remarkable number of patients with CD reporting that the risk for developing complicated disease phenotype is not different between pediatric onset and adult onset CD patients.
P- Reviewers Karagiannis S, Pehl C S- Editor Song XX L- Editor A E- Editor Zhang DN
| 1. | Lees CW, Barrett JC, Parkes M, Satsangi J. New IBD genetics: common pathways with other diseases. Gut. 2011;60:1739-1753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 406] [Cited by in F6Publishing: 431] [Article Influence: 33.2] [Reference Citation Analysis (3)] |
| 2. | Lakatos PL. Recent trends in the epidemiology of inflammatory bowel diseases: up or down? World J Gastroenterol. 2006;12:6102-6108. [PubMed] [Cited in This Article: ] |
| 3. | Tarrant KM, Barclay ML, Frampton CM, Gearry RB. Perianal disease predicts changes in Crohn’s disease phenotype-results of a population-based study of inflammatory bowel disease phenotype. Am J Gastroenterol. 2008;103:3082-3093. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 4. | Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C, Geboes K. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5-36. [PubMed] [Cited in This Article: ] |
| 5. | Louis E, Collard A, Oger AF, Degroote E, Aboul Nasr El Yafi FA, Belaiche J. Behaviour of Crohn’s disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001;49:777-782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 684] [Cited by in F6Publishing: 674] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 6. | Cosnes J, Cattan S, Blain A, Beaugerie L, Carbonnel F, Parc R, Gendre JP. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis. 2002;8:244-250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 981] [Cited by in F6Publishing: 930] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 7. | Lakatos PL, Czegledi Z, Szamosi T, Banai J, David G, Zsigmond F, Pandur T, Erdelyi Z, Gemela O, Papp J. Perianal disease, small bowel disease, smoking, prior steroid or early azathioprine/biological therapy are predictors of disease behavior change in patients with Crohn’s disease. World J Gastroenterol. 2009;15:3504-3510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 83] [Cited by in F6Publishing: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Solberg IC, Vatn MH, Høie O, Stray N, Sauar J, Jahnsen J, Moum B, Lygren I. Clinical course in Crohn’s disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol. 2007;5:1430-1438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 502] [Cited by in F6Publishing: 502] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 9. | Bernstein CN, Loftus EV, Ng SC, Lakatos PL, Moum B. Hospitalisations and surgery in Crohn’s disease. Gut. 2012;61:622-629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 218] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 10. | Abraham BP, Mehta S, El-Serag HB. Natural history of pediatric-onset inflammatory bowel disease: a systematic review. J Clin Gastroenterol. 2012;46:581-589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 11. | Pigneur B, Seksik P, Viola S, Viala J, Beaugerie L, Girardet JP, Ruemmele FM, Cosnes J. Natural history of Crohn’s disease: comparison between childhood- and adult-onset disease. Inflamm Bowel Dis. 2010;16:953-961. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 196] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 12. | de Bie CI, Paerregaard A, Kolacek S, Ruemmele FM, Koletzko S, Fell JM, Escher JC; and the EUROKIDS Porto IBD Working Group of ESPGHAN. Disease Phenotype at Diagnosis in Pediatric Crohn’s Disease: 5-year Analyses of the EUROKIDS Registry. Inflamm Bowel Dis. 2013;19:378-385. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Levine A. Pediatric inflammatory bowel disease: is it different? Dig Dis. 2009;27:212-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Van Limbergen J, Russell RK, Drummond HE, Aldhous MC, Round NK, Nimmo ER, Smith L, Gillett PM, McGrogan P, Weaver LT. Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology. 2008;135:1114-1122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 632] [Cited by in F6Publishing: 650] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 15. | Lakatos L, Kiss LS, David G, Pandur T, Erdelyi Z, Mester G, Balogh M, Szipocs I, Molnar C, Komaromi E. Incidence, disease phenotype at diagnosis, and early disease course in inflammatory bowel diseases in Western Hungary, 2002-2006. Inflamm Bowel Dis. 2011;17:2558-2565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 16. | Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989;170:2-6; discussion 16-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1396] [Cited by in F6Publishing: 1429] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 17. | IBD Working Group of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition. Inflammatory bowel disease in children and adolescents: recommendations for diagnosis--the Porto criteria. J Pediatr Gastroenterol Nutr. 2005;41:1-7. [PubMed] [Cited in This Article: ] |
| 18. | Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, Ochsenkühn T, Orchard T, Rogler G, Louis E. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Definitions and diagnosis. J Crohns Colitis. 2010;4:7-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 818] [Cited by in F6Publishing: 780] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 19. | Vernier-Massouille G, Balde M, Salleron J, Turck D, Dupas JL, Mouterde O, Merle V, Salomez JL, Branche J, Marti R. Natural history of pediatric Crohn’s disease: a population-based cohort study. Gastroenterology. 2008;135:1106-1113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 427] [Cited by in F6Publishing: 434] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 20. | Mahid SS, Minor KS, Stevens PL, Galandiuk S. The role of smoking in Crohn’s disease as defined by clinical variables. Dig Dis Sci. 2007;52:2897-2903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Picco MF, Bayless TM. Tobacco consumption and disease duration are associated with fistulizing and stricturing behaviors in the first 8 years of Crohn’s disease. Am J Gastroenterol. 2003;98:363-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 22. | Cosnes J. Tobacco and IBD: relevance in the understanding of disease mechanisms and clinical practice. Best Pract Res Clin Gastroenterol. 2004;18:481-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 158] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 23. | Aldhous MC, Drummond HE, Anderson N, Smith LA, Arnott ID, Satsangi J. Does cigarette smoking influence the phenotype of Crohn’s disease? Analysis using the Montreal classification. Am J Gastroenterol. 2007;102:577-588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 24. | Cosnes J, Carbonnel F, Beaugerie L, Le Quintrec Y, Gendre JP. Effects of cigarette smoking on the long-term course of Crohn’s disease. Gastroenterology. 1996;110:424-431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 282] [Cited by in F6Publishing: 267] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 25. | Szamosi T, Banai J, Lakatos L, Czegledi Z, David G, Zsigmond F, Pandur T, Erdelyi Z, Gemela O, Papp M. Early azathioprine/biological therapy is associated with decreased risk for first surgery and delays time to surgery but not reoperation in both smokers and nonsmokers with Crohn’s disease, while smoking decreases the risk of colectomy in ulcerative colitis. Eur J Gastroenterol Hepatol. 2010;22:872-879. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Loly C, Belaiche J, Louis E. Predictors of severe Crohn’s disease. Scand J Gastroenterol. 2008;43:948-954. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 209] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 27. | Beaugerie L, Seksik P, Nion-Larmurier I, Gendre JP, Cosnes J. Predictors of Crohn’s disease. Gastroenterology. 2006;130:650-656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 630] [Cited by in F6Publishing: 623] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 28. | Lakatos PL, Golovics PA, David G, Pandur T, Erdelyi Z, Horvath A, Mester G, Balogh M, Szipocs I, Molnar C. Has there been a change in the natural history of Crohn’s disease? Surgical rates and medical management in a population-based inception cohort from Western Hungary between 1977-2009. Am J Gastroenterol. 2012;107:579-588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 175] [Article Influence: 14.6] [Reference Citation Analysis (0)] |