Copyright
©2013 Baishideng Publishing Group Co.
World J Gastroenterol. Mar 21, 2013; 19(11): 1707-1717
Published online Mar 21, 2013. doi: 10.3748/wjg.v19.i11.1707
Published online Mar 21, 2013. doi: 10.3748/wjg.v19.i11.1707
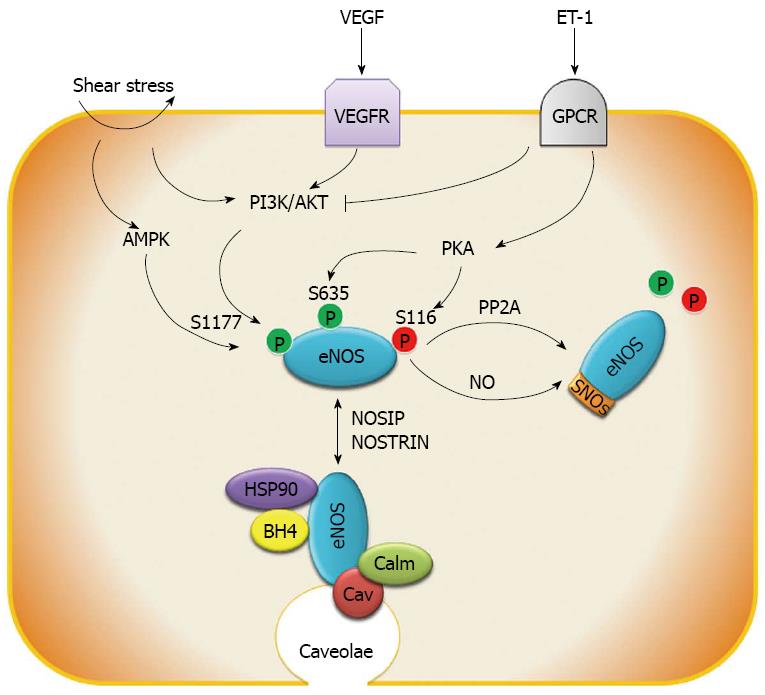
Figure 2 The molecular regulation of endothelial nitric oxide synthase activity.
Endothelial nitric oxide synthase (eNOS) phosphorylation can be triggered by shear stress, vascular endothelial growth factor (VEGF), endothelin 1 (ET-1) and other factors though adenosine monophosphate-activated protein kinase (AMPK), protein kinase B (AKT) and protein kinase A (PKA) pathways, whereas protein phosphatase 2 (PP2A) de-phosphorylates eNOS. In addition, S-nitrosylation (SNOs) by eNOS-derived nitric oxide (NO) inhibits eNOS activity. Endothelial nitric oxide synthase interacting protein (NOSIP) and endothelial nitric oxide synthase trafficking inducer protein (NOSTRIN) regulate the sub-cellular location of eNOS protein between the caveolae and cytoplasm. The principal location of eNOS is in caveolae where its function is inhibited by binding to caveolin (Cav). HSP90, calmodulin (Calm) and tetrahydrobiopterin (BH4) are indispensable proteins and cofactors for catalyzing NO production. PI3K: Phosphatidylinositol-3-kinase; GPCR: G protein-coupled receptor.
- Citation: Hu LS, George J, Wang JH. Current concepts on the role of nitric oxide in portal hypertension. World J Gastroenterol 2013; 19(11): 1707-1717
- URL: https://www.wjgnet.com/1007-9327/full/v19/i11/1707.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i11.1707