Published online Mar 14, 2013. doi: 10.3748/wjg.v19.i10.1618
Revised: January 21, 2013
Accepted: February 5, 2013
Published online: March 14, 2013
AIM: To investigate the influence of percutaneous local therapy on gastric myoelectrical activity in patients with hepatocellular carcinomas.
METHODS: Forty-four patients with hepatocellular carcinoma (HCC) [27 males and 17 females, ranging in age from 49 to 81 years old (69.7 ± 8.01 years)] who were admitted for percutaneous local therapy were enrolled in this study. We examined clinical abdominal symptoms using the Gastrointestinal Symptom Rating Scale (GSRS) before and 3 d after percutaneous local therapy. We also measured cutaneous fasting and postprandial electrogastrography (EGG) recordings before and 3 d after percutaneous local therapy.
RESULTS: We found that the percentage of normogastria in the fasting period was lower in the Child B group than in the Child A group (66.8% ± 8.6% vs 84.0% ± 3.8%). After percutaneous local therapy for HCC, the percentages of normogastria in the fasting period were significantly decreased (81.6% ± 3.5% vs 75.2% ± 4.5%). None of the postprandial EGG parameters changed significantly after percutaneous local therapy for HCC. Percutaneous local therapy for HCC reduced the power ratio (PR). In particular, the PR of tachygastria was significantly decreased after therapy (P < 0.01). However, no significant differences were found in the postprandial EGG parameters. Likewise, no significant differences were found in the calculated GSRS scores obtained from the questionnaire before and after therapy.
CONCLUSION: Gastric slow-wave dysrhythmias were induced by percutaneous local therapy in HCC patients, even though the GSRS scores obtained from the questionnaire did not change significantly.
- Citation: Kobayashi M, Kinekawa F, Matsuda K, Fujihara S, Nishiyama N, Nomura T, Tani J, Miyoshi H, Kobara H, Deguchi A, Yoneyama H, Mori H, Masaki T. Influence of percutaneous local therapy for hepatocellular carcinoma on gastric function. World J Gastroenterol 2013; 19(10): 1618-1624
- URL: https://www.wjgnet.com/1007-9327/full/v19/i10/1618.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i10.1618
In treating hepatocellular carcinoma (HCC), various percutaneous local therapies, such as percutaneous ethanol injection (PEI), microwave coagulation, and radio frequency ablation (RFA), are frequently used because of their demonstrated effectiveness. Although these treatment modalities can induce coagulated tumor necrosis effectively, they are also known to cause adverse effects on extrahepatic abdominal organs[1]. There are, however, few published reports on the influence of percutaneous local therapy on gastric myenteric activity. Therefore, it is unclear whether gastric function is affected by percutaneous local therapy.
Gastric myoelectrical activity is regulated by electrical pacemaker activity known as slow waves. Gastric slow waves originating from pacemaker cells on the major curvature of the stomach can be measured noninvasively by using a cutaneous electrogastrography (EGG) recorder and placing electrodes on the abdominal skin. In this study, to clarify the influence of percutaneous local therapy for HCC on gastric function, we continuously recorded gastric myoelectric activity by EGG and estimated the influence of percutaneous local therapy on HCC gastric function. In addition, we investigated whether gastric slow-wave dysrhythmias were associated with clinical abdominal symptoms occurring after therapy.
Forty-four patients with HCC [27 males and 17 females, ranging in age from 49 to 81 years old (69.7 ± 8.01 years)] were enrolled in the present study. Patients with diseases known to affect gastric myoelectrical activity, such as diabetes mellitus, who had received a partial or total gastrectomy or who were taking medication known to alter gastrointestinal electrical activity were excluded from this study. After being provided with a careful explanation regarding the goals of the investigation, all patients provided informed consent. The study was conducted in accordance with the local ethical guidelines and the recommendations of the Declaration of Helsinki. HCC was diagnosed by its characteristic appearance on ultrasonography, computed tomographic scan, angiography, serum a-fetoprotein assays, and serum protein induced by vitamin K absence or antagonist-2 assays.
We have developed several novel percutaneous local therapies for HCC. The first of these therapies is the combination of PEI and RFA (PEI-RFA). In this treatment modality, RFA is performed immediately after PEI. The second therapy is percutaneous ethanol-lipiodol injection (PELI). In this modality, a 10:1 mixture of pure ethanol and lipiodol, a lipid-based contrast medium, is injected percutaneously into the HCC. The third therapy is the combination of PELI and RFA (PELI-RFA). The final therapy is RFA alone. The relative usefulness of each of these new treatment modalities has been reported elsewhere[2-8]. In the present study, 18 patients with HCC underwent PEI-RFA, 7 patients underwent PELI, 6 patients underwent PELI-RFA, and 13 patients underwent RFA.
We examined clinical abdominal symptoms using the questionnaire developed by Svedlund et al[9] (Gastrointestinal Symptom Rating Scale, GSRS), which was translated into Japanese. The GSRS consists of 15 items that assess symptoms of the digestive tract on an interview-based rating scale. Items on the GSRS are scored using a seven-point scale, where a score of 1 represents the absence of any troublesome symptoms and a score of 7 represents very troublesome symptoms. The 15 items on the GSRS evaluate the following 5 symptom categories: reflux (heartburn and regurgitation), abdominal pain (abdominal pain, hunger pains, and nausea), diarrhea (diarrhea, loose stools, and an urgent need to defecate), indigestion (borborygmus, abdominal distension, eructation, and increased flatus), and constipation (constipation, hard stools, and a feeling of incomplete evacuation.
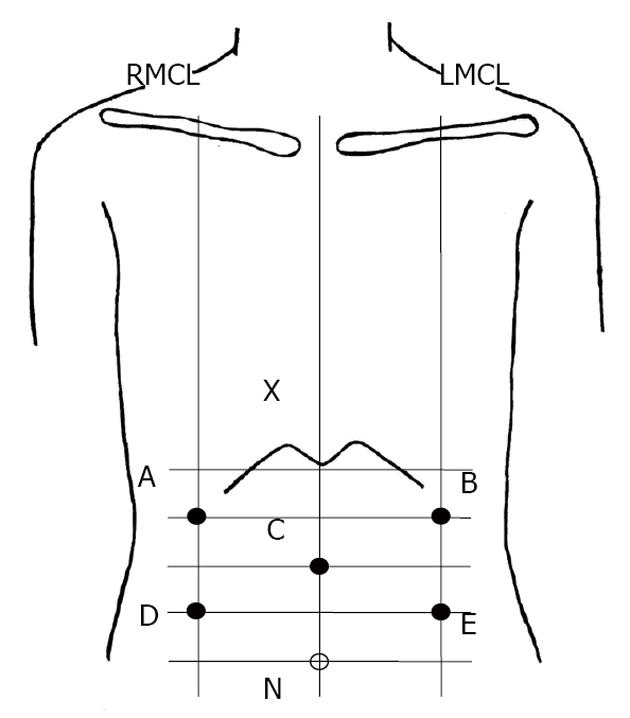
We recorded an EGG before therapy began and on the third day after therapy and compared the results. The EGGs were recorded with a portable electrogastrographic recorder (NIPRO, EGG, A and D, Tokyo, Japan). The central terminal electrode for the EGG recorder was placed at the midpoint between the processus xiphoideus and the navel, and 4 other electrodes were placed above, below, and to the left and right of the stomach (Figure 1). The recordings of the EGGs were stable because of the tenth filter sampling at 1-s cycles. Data recording was performed at 13 bits and at a frequency between 2.1 and 6.0 cycles/min. Electrogastrography was performed with the patients in a dorsal position. Patients were asked to remain as still as possible to reduce motion artifacts. Electrogastrograms were recorded for 30 min during a fasting period and again during a postprandial period.
The EGG data were downloaded to a personal computer via an RS-232C port, and a frequency analysis (fast Fourier transformation; FFT analysis) was performed on 512 points and analyzed using EGS2 Ver.1.31 software (Gram, Japan). Using an FFT analysis, we evaluated the percentages of bradygastria (< 2.4 cycles/min), normogastria (2.4-3.6 cycles/min), and tachygastria (> 3.6 cycles/min), as well as the dominant frequency and the postprandial-to-fasting power ratio (PR).
Measured values are expressed as the mean ± SE. Comparisons before and after therapy were performed by the paired Student t test, and P < 0.05 was accepted as indicating a significant difference. A regression analysis was performed to examine the relationship between decreases in the percentage of normogastria (%normogastria) after treatment and clinical factors. A P value < 0.05 was considered statistically significant. The software package used for the statistical analysis was SPSS (SPSS statistics 20 IBM Corporation, New York, United States).
According to the Child-Pugh classification of liver disease severity, there were 33 patients in stage A and 11 patients in stage B (Table 1), and the conditions of their Child-Pugh classifications remained the same during the administration of percutaneous local therapy. The causes of liver dysfunction in the HCC patients were as follows: chronic hepatitis B virus infection in 5 cases, chronic hepatitis C virus infection in 30 cases, negative for both hepatitis B surface antigen and hepatitis C antibody in 5 cases, alcoholism in 3 cases, and primary biliary cirrhosis in 1 case.
| Sex (male/female) | 27/17 |
| Age (yr), means ± SD | 69.7 ± 8.0 |
| Cause of liver dysfunction | |
| HBV | 5 |
| HCV | 30 |
| Alcoholic | 3 |
| PBC | 1 |
| NBNC | 5 |
| Child-Pugh A/B | 33/11 |
| Therapy | |
| PELI | 7 |
| PELI-RFA | 6 |
| PEI-RFA | 18 |
| RFA | 13 |
No major complications occurred during or after treatment. All the subjects tolerated the electrogastrographic examination well, and no gastrointestinal complaints were reported by the patients during the EGG recording. First, we examined the gastric myenteric activity among the liver cirrhosis subgroups before the treatment. The clinical characteristics of the patients in the Child A and Child B group are shown in Table 2, and the pretreatment EGG data are shown in Table 3.
| Child A group | Child B group | |
| Sex (male/female) | 23/10 | 4/7 |
| Age (yr) | 69.4 ± 8.0 | 70.6 ± 8.8 |
| Cause of liver dysfunction | ||
| HBV | 4 | 1 |
| HCV | 23 | 7 |
| Alcoholic | 2 | 1 |
| PBC | 0 | 1 |
| NBNC | 4 | 1 |
| Child A (n = 33) | Child B (n = 11) | P value | Before | After | P value | |
| Fasting EGG | ||||||
| DF (circles/min) | 2.89 ± 0.08 | 2.73 ± 0.18 | NS | 2.89 ± 0.07 | 2.84 ± 0.08 | NS |
| Normogastria | 84.0% ± 3.8% | 66.8% ± 8.6% | < 0.05 | 81.6% ± 3.5% | 75.2% ± 4.5% | < 0.05 |
| Bradygastria | 14.8% ± 3.8% | 30.1% ± 8.8% | NS | 16.6% ± 3.5% | 23.2% ± 4.3% | NS |
| Tachygastria | 1.7% ± 5.5% | 1.6% ± 6.1% | NS | 1.7% ± 0.9% | 1.6% ± 0.9% | NS |
| Post-meal EGG | ||||||
| DF (circles/min) | 2.53 ± 0.09 | 2.56 ± 0.14 | NS | 2.55 ± 0.08 | 2.67 ± 0.08 | NS |
| Normogastria | 54.9% ± 4.8% | 51.7% ± 8.3% | NS | 54.9% ± 4.3% | 53.8% ± 4.3% | NS |
| Bradygastria | 43.2% ± 4.8% | 40.5% ± 8.2% | NS | 41.5% ± 4.3% | 37.5% ± 4.0% | NS |
| Tachygastria | 1.9% ± 1.0% | 7.8% ± 4.8% | NS | 3.6% ± 1.5% | 8.7% ± 3.0% | NS |
| PR of bradygastria | 2.3 ± 0.4 | 2.2 ± 0.8 | NS | 2.3 ± 0.4 | 1.9 ± 0.2 | NS |
| PR of normogastria | 1.4 ± 0.2 | 1.4 ± 0.3 | NS | 1.4 ± 0.2 | 1.4 ± 0.1 | NS |
| PR of tachygastria | 2.2 ± 0.3 | 2.3 ± 0.7 | NS | 2.2 ± 0.3 | 0.7 ± 0.1 | < 0.01 |
We found that the percentage of normogastria in the fasting period was lower in the Child B group than in the Child A group. However, no significant differences were found in the calculated GSRS scores obtained from the questionnaire. Furthermore, no differences in symptom categories were significant (Table 4).
| Child A | Child B | P value | Before | After | P value | |
| GSRS score | 1.5 ± 0.1 | 1.7 ± 0.3 | NS | 1.5 ± 0.1 | 1.6 ± 0.1 | NS |
| Reflux | 1.4 ± 0.1 | 1.4 ± 0.3 | NS | 1.4 ± 0.1 | 1.4 ± 0.2 | NS |
| Abdominal pain | 1.3 ± 0.1 | 1.6 ± 0.2 | NS | 1.4 ± 0.1 | 1.6 ± 1.2 | NS |
| Indigestion | 1.4 ± 0.1 | 1.9 ± 0.3 | NS | 1.6 ± 0.1 | 1.6 ± 0.1 | NS |
| Diarrhea | 1.3 ± 0.1 | 1.5 ± 0.2 | NS | 1.4 ± 0.1 | 1.6 ± 0.1 | NS |
| Constipation | 1.8 ± 0.2 | 2.2 ± 0.4 | NS | 1.9 ± 0.2 | 1.9 ± 0.2 | NS |
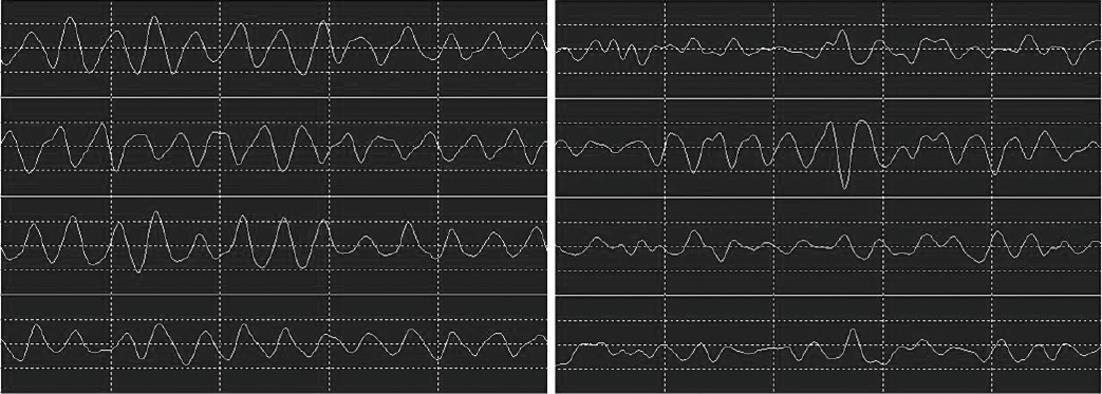
Next, we examined the change in EGG associated with treatment. The EGG results are summarized in Table 3. Examples of the raw EGG signals before and after therapy are shown in Figure 2.
After percutaneous local therapy for HCC, the percentages of normogastria in the fasting period were significantly decreased (P < 0.05). Although the ratio of bradygastria increased, the change was not significant (P = 0.967). None of the postprandial EGG parameters changed significantly after percutaneous local therapy for HCC. Therapy reduced the PR of bradygastria, normogastria, and tachygastria. In particular, the PR of tachygastria was significantly decreased after therapy (P < 0.01).
Conversely, no significant differences were found in the calculated GSRS scores changes within any symptom category (Table 4). A single regression analysis was performed to examine the relationship between decreases in the percentage of normogastria (%normogastria) after treatment and the following factors: patient sex, patient age, ethanol injection, location of the treated hepatoma (right or left lobe), tumor size (diameter), use of RFA, and the Child-Pugh score. The results revealed that the decreases in %normogastria were positively correlated with patient age (P = 0.037). Moreover, the decreases in %normogastria were significantly larger in the group with ethanol consumption than in the group without ethanol consumption (P = 0.018). A multiple regression analysis revealed, however, that none of the independent variables were significantly related to the decreases in %normogastria. In assessing the effect of the independent variables based on P value, tumor size appeared to have the strongest influence on the changes in %normogastria (Table 5).
| Regression coefficient | 95%CI | P value | Partial regression coefficient | 95%CI | P value | |||
| Sex (M) | -10.77 | -27.34 | 5.79 | 0.196 | -11.58 | -29.4 | 6.25 | 0.196 |
| Age(yr) | 1.05 | 0.07 | 2.04 | 0.037 | 0.73 | -0.45 | 1.91 | 0.22 |
| Ethanol (use) | 20.6 | 3.75 | 37.46 | 0.018 | 13.99 | -3.88 | 31.87 | 0.121 |
| Tumor location (left lobe) | -12.26 | -32.3 | 7.78 | 0.224 | -11.83 | -32.05 | 8.39 | 0.243 |
| Tumor size (cm) | -5.78 | -14.32 | 2.77 | 0.18 | -6.6 | -14.89 | 1.68 | 0.115 |
| RFA (use) | -0.54 | -23.04 | 21.96 | 0.962 | -5.74 | -27.61 | 16.13 | 0.598 |
| Child-Pugh (score) | -4.52 | -11.96 | 2.92 | 0.227 | -8.05 | -15.3 | -0.8 | 0.031 |
We previously reported the influence of percutaneous local therapy for HCC on gastric myenteric activity[10]. This is the first formal report with additional cases.
The first electrogastrogram measurement in humans was performed by Alvarez, who found that ECG waves recorded on the body surface of a middle-aged woman occurred at a rate of three cycles per minute.
This method can be used to noninvasively assess the electrical activity generated by gastric smooth muscles[11].
Although the role of EGG in clinical gastroenterology has not yet been clearly defined and the cause of gastric dysrhythmias at the cellular level is unknown, EGGs are believed to reflect the electrical control activity and gastric motility regulated by pacemakers. In humans, these EGG waves originate from the pacemaker area along the major curvature of the stomach and propagate aborally with increasing velocity at intervals of approximately 20 s. EGGs have been shown to provide useful information for clinical diagnoses.
Abnormal gastric slow-wave frequencies have been described in disorders of gastric emptying, nausea and vomiting in pregnancy[12], motion sickness[13], anorexia nervosa[14], functional dyspepsia[15-18], and diabetic gastroparesis[19-21].
Patients with cirrhosis of the liver frequently present with many gastrointestinal complaints that are most likely due to abnormal gastrointestinal motility.
However, reports on the gastric myoelectrical activity in liver diseases are surprisingly scarce, with only a few papers. Caras et al[22] demonstrated an abnormal electrogastrogram in 8 of 14 (57%) patients with end-stage liver disease awaiting liver transplants. Usami et al[23] found a decreased share of the power within the normogastria-range relative to the total power of the entire frequency band considered in a study group comprising 32 patients with liver cirrhosis of various etiologies and stages.
Their study also confirmed that the results of EGG became less favorable as the severity of liver damage increased.
Gastrointestinal hormones may increase in liver cirrhosis owing to reduced hepatic metabolism and portosystemic shunting. Metabolic abnormalities of these peptides have been reported in patients with liver cirrhosis.
In some studies, the levels of plasma vasoactive peptides with liver cirrhosis have been shown to be higher compared with the normal population[24,25].
It has been shown that transcatheter arterial chemoembolization affects gastric myenteric activity and that overproduction of endogenous prostaglandin is related to dysrhythmia of the gastric myenteric activity, suggesting that prostaglandin is related to the activation of the inflammatory response, production of pain, and fever[26].
Pain and stress may affect the stomach’s electrical and mechanical activities. Therefore, it is useful to know whether the abdominal pain induced by percutaneous local therapy produces changes in gastric myoelectric activity.
In this study, multiple regression analysis revealed that tumor size was the strongest and has strong adverse effects on gastric motility.
The pathophysiology of gastric dysrhythmias induced by percutaneous local therapy in HCC patients is poorly understood.
In this study, we demonstrated that gastric myenteric activity was affected by percutaneous local therapy for HCC, even though abdominal symptoms were not apparent and the GSRS scores obtained from the questionnaire did not change significantly after therapy. Delayed gastric transit is a significant clinical matter that may occur after percutaneous therapy for HCC. Because patients with HCC have reportedly tended to have gastrointestinal dysfunction[27], we must monitor gastrointestinal dysfunction after percutaneous local therapy for HCC even when there no clinical symptoms are present.
We found that the percentage of normogastria during fasting was decreased after therapy, suggesting that therapy may have an adverse effect on the interdigestive migrating contractions (IMCs) of the fasting period.
IMCs occur approximately every two hours in humans. The physiological role of IMCs is thought to be the housekeeping of the gastrointestinal tract by transferring food residues and detached epithelium toward the rectum through intense contractions that migrate from the stomach to the end of the ileum[28]. IMCs have four phases, as described by Szurszewski[29], and there are two types of IMCs. IMCs originating in the stomach are called gastrointestinal IMCs (GI-IMCs), and those originating in the duodenum or lower tract are called intestinal IMCs (I-IMCs). Motilin, which is secreted by the duodenum and upper intestinal mucosa, triggers GI-IMC but is not involved in I-IMCs[30]. In recent years, ghrelin and serotonin in the digestive tract have been of interest as mediators of IMCs[31-33].
Normal gastrointestinal movement is also involved in immunity. The secretion of immunoglobulin A peaks during IMCs in the intestinal tract[34], and a lack of housekeeping by IMCs induces bacterial overgrowth (BO)[35]. Patients with hepatic cirrhosis are known to have prolonged orocecal transit times and are likely to suffer from small intestine BO[36]. As shown in animal models, aggravated BO may allow bacterial translocation and the development of sepsis. Thus, maintaining normal gastrointestinal movement is of clinical significance in patients with hepatic cirrhosis who are susceptible to infection.
In conclusion, gastric slow-wave dysrhythmias were induced by percutaneous local therapy in HCC patients, but no significant differences were found in the calculated GSRS scores obtained from patient questionnaires collected before and after therapy. The mechanisms underlying the effect of percutaneous local therapy for HCC on extrahepatic abdominal organs require further exploration.
In the treatment of hepatocellular carcinoma (HCC), various percutaneous local therapies are frequently used, but they are also known to cause adverse effects on extrahepatic abdominal organs. However, the influence of percutaneous local therapy on gastric myenteric activity has not been well investigated.
Because patients with HCC tend to have gastrointestinal dysfunction, we must monitor gastrointestinal dysfunction after percutaneous local therapy for HCC. In this study, the authors continuously recorded the gastric myoelectric activity by electrogastrography (EGG) and estimated the influence of percutaneous local therapy on HCC gastric function to clarify the influence of percutaneous local therapy for HCC on gastric function.
There are few published reports on the influence of percutaneous local therapy on gastric myenteric activity. To the best of the authors’ knowledge, the present study was the most detailed and systematic study investigating the influence of percutaneous local therapy on gastric myoelectrical activity in patients with HCC.
Gastric slow-wave dysrhythmias were induced by percutaneous local therapy in HCC patients, even though abdominal symptoms were not apparent and the Gastrointestinal Symptom Rating Scale scores obtained from the questionnaire did not change significantly after therapy.
EGG is a method that enables gastric electrical activity to be recorded using abdominal surface electrodes. The method has the advantages of being noninvasive, convenient, and providing considerable functional information.
This is a very well organized and well conducted study. The results are clearly described, and the discussion and conclusions do not overstate the results.
P- Reviewer Fisher RA S- Editor Gou SX L- Editor A E- Editor Xiong L
| 1. | Koda M, Murawaki Y, Hirooka Y, Kitamoto M, Ono M, Sakaeda H, Joko K, Sato S, Tamaki K, Yamasaki T. Complications of radiofrequency ablation for hepatocellular carcinoma in a multicenter study: An analysis of 16346 treated nodules in 13283 patients. Hepatol Res. 2012;42:1058-1064. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 2. | Kurokohchi K, Watanabe S, Masaki T, Hosomi N, Funaki T, Arima K, Yoshida S, Nakai S, Murota M, Miyauchi Y. Combination therapy of percutaneous ethanol injection and radiofrequency ablation against hepatocellular carcinomas difficult to treat. Int J Oncol. 2002;21:611-615. [PubMed] [Cited in This Article: ] |
| 3. | Kurokohchi K, Watanabe S, Masaki T, Hosomi N, Funaki T, Arima K, Yoshida S, Miyauchi Y, Kuriyama S. Combined use of percutaneous ethanol injection and radiofrequency ablation for the effective treatment of hepatocelluar carcinoma. Int J Oncol. 2002;21:841-846. [PubMed] [Cited in This Article: ] |
| 4. | Watanabe S, Kurokohchi K, Masaki T, Miyauchi Y, Funaki T, Inoue H, Himoto T, Kita Y, Uchida N, Touge T. Enlargement of thermal ablation zone by the combination of ethanol injection and radiofrequency ablation in excised bovine liver. Int J Oncol. 2004;24:279-284. [PubMed] [Cited in This Article: ] |
| 5. | Kurokohchi K, Masaki T, Miyauchi Y, Funaki T, Yoneyama H, Miyoshi H, Yoshida S, Himoto T, Morishita A, Uchida N. Percutaneous ethanol and lipiodol injection therapy for hepatocellular carcinoma. Int J Oncol. 2004;24:381-387. [PubMed] [Cited in This Article: ] |
| 6. | Kurokohchi K, Masaki T, Miyauchi Y, Hosomi N, Yoneyama H, Yoshida S, Himoto T, Deguchi A, Nakai S, Inoue H. Efficacy of combination therapies of percutaneous or laparoscopic ethanol-lipiodol injection and radiofrequency ablation. Int J Oncol. 2004;25:1737-1743. [PubMed] [Cited in This Article: ] |
| 7. | Kurokohchi K, Masaki T, Himoto T, Deguchi A, Nakai S, Yoneyama H, Yoshida S, Kimura Y, Inoue H, Kinekawa F. Successful laparoscopic radiofrequency ablation of hepatocellular carcinoma adhered to the mesentery after transcatheter arterial embolization. Oncol Rep. 2005;13:65-68. [PubMed] [Cited in This Article: ] |
| 8. | Kurokohchi K, Watanabe S, Masaki T, Hosomi N, Miyauchi Y, Himoto T, Kimura Y, Nakai S, Deguchi A, Yoneyama H. Comparison between combination therapy of percutaneous ethanol injection and radiofrequency ablation and radiofrequency ablation alone for patients with hepatocellular carcinoma. World J Gastroenterol. 2005;11:1426-1432. [PubMed] [Cited in This Article: ] |
| 9. | Svedlund J, Sjödin I, Dotevall G. GSRS--a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. 1988;33:129-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 884] [Cited by in F6Publishing: 928] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 10. | Alvarez WC. The electrogastrogram and what it shows. JAMA. 1922;78:1116-1119. [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 220] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Kinekawa F, Matsuda K, Masaki T, Kurokohchi K, Yoneyama H, Inoue H, Kurata H, Uchida Y, Watanabe S, Kuriyama S. Percutaneous local therapies for hepatocellular carcinoma impair gastric function. World J Gastroenterol. 2006;12:157-158. [PubMed] [Cited in This Article: ] |
| 12. | Riezzo G, Pezzolla F, Darconza G, Giorgio I. Gastric myoelectrical activity in the first trimester of pregnancy: a cutaneous electrogastrographic study. Am J Gastroenterol. 1992;87:702-707. [PubMed] [Cited in This Article: ] |
| 13. | Stern RM, Koch KL, Leibowitz HW, Lindblad IM, Shupert CL, Stewart WR. Tachygastria and motion sickness. Aviat Space Environ Med. 1985;56:1074-1077. [PubMed] [Cited in This Article: ] |
| 14. | Ravelli AM, Helps BA, Devane SP, Lask BD, Milla PJ. Normal gastric antral myoelectrical activity in early onset anorexia nervosa. Arch Dis Child. 1993;69:342-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Lin Z, Eaker EY, Sarosiek I, McCallum RW. Gastric myoelectrical activity and gastric emptying in patients with functional dyspepsia. Am J Gastroenterol. 1999;94:2384-2389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 80] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Leahy A, Besherdas K, Clayman C, Mason I, Epstein O. Abnormalities of the electrogastrogram in functional gastrointestinal disorders. Am J Gastroenterol. 1999;94:1023-1028. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 89] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Lu CL, Chen CY, Chang FY, Kang LJ, Lee SD, Wu HC, Kuo TS. Impaired postprandial gastric myoelectrical activity in Chinese patients with nonulcer dyspepsia. Dig Dis Sci. 2001;46:242-249. [PubMed] [Cited in This Article: ] |
| 18. | Lin X, Chen JZ. Abnormal gastric slow waves in patients with functional dyspepsia assessed by multichannel electrogastrography. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1370-G1375. [PubMed] [Cited in This Article: ] |
| 19. | Koch KL, Stern RM, Stewart WR, Vasey MW. Gastric emptying and gastric myoelectrical activity in patients with diabetic gastroparesis: effect of long-term domperidone treatment. Am J Gastroenterol. 1989;84:1069-1075. [PubMed] [Cited in This Article: ] |
| 20. | Rothstein RD, Alavi A, Reynolds JC. Electrogastrography in patients with gastroparesis and effect of long-term cisapride. Dig Dis Sci. 1993;38:1518-1524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 92] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Hongo M, Okuno Y. Diabetic gastropathy in patients with autonomic neuropathy. Diabet Med. 1993;10 Suppl 2:79S-81S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Caras SD, Dickson RC, Lin Z, Ishitani MB, Caldwell SH, Chen JD. Gastric myoelectric activity in patients with end-stage liver disease. Scand J Gastroenterol. 1999;34:883-888. [PubMed] [Cited in This Article: ] |
| 23. | Usami A, Mizukami Y, Onji M. Abnormal gastric motility in liver cirrhosis: roles of secretin. Dig Dis Sci. 1998;43:2392-2397. [PubMed] [Cited in This Article: ] |
| 24. | Grassi M, Albiani B, De Matteis A, Fontana M, Lucchetta MC, Raffa S. [Prevalence of dyspepsia in liver cirrhosis: a clinical and epidemiological investigation]. Minerva Med. 2001;92:7-12. [PubMed] [Cited in This Article: ] |
| 25. | Richter JE. Role of the gastric refluxate in gastroesophageal reflux disease: acid, weak acid and bile. Am J Med Sci. 2009;338:89-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Chang CS, Yang SS, Yeh HZ, Ko CW, Lien HC, Chen GH. Mediation of transcatheter arterial chemoembolization induced gastric slow-wave dysrhythmia by endogenous prostaglandin. J Gastroenterol Hepatol. 2002;17:46-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Chen CY, Lu CL, Chang FY, Lih-Jiun K, Luo JC, Lu RH, Lee SD. Delayed gastrointestinal transit in patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 2002;17:1254-1259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Itoh Z, Honda R, Hiwatashi K, Takeuchi S, Aizawa I, Takayanagi R, Couch EF. Motilin-induced mechanical activity in the canine alimentary tract. Scand J Gastroenterol Suppl. 1976;39:93-110. [PubMed] [Cited in This Article: ] |
| 29. | Szurszewski JH. A migrating electric complex of canine small intestine. Am J Physiol. 1969;217:1757-1763. [PubMed] [Cited in This Article: ] |
| 30. | Kusano M, Sekiguchi T, Nishioka T, Kawamura O, Kikuchi K, Matsuzaki T, Horikoshi T, Kobayashi S. The relationship between interdigestive gallbladder and gastroduodenal motility in man. Gastroenterol Jpn. 1990;25:568-574. [PubMed] [Cited in This Article: ] |
| 31. | Tack J, Depoortere I, Bisschops R, Delporte C, Coulie B, Meulemans A, Janssens J, Peeters T. Influence of ghrelin on interdigestive gastrointestinal motility in humans. Gut. 2006;55:327-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 276] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 32. | Nakajima H, Mochiki E, Zietlow A, Ludwig K, Takahashi T. Mechanism of interdigestive migrating motor complex in conscious dogs. J Gastroenterol. 2010;45:506-514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Zietlow A, Nakajima H, Taniguchi H, Ludwig K, Takahashi T. Association between plasma ghrelin and motilin levels during MMC cycle in conscious dogs. Regul Pept. 2010;164:78-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Fändriks L, Mattsson A, Dalenbäck J, Sjövall H, Olbe L, Svennerholm AM. Gastric output of IgA in man: relation to migrating motility complexes and sham feeding. Scand J Gastroenterol. 1995;30:657-663. [PubMed] [Cited in This Article: ] |
| 35. | Vantrappen G, Janssens J, Hellemans J, Ghoos Y. The interdigestive motor complex of normal subjects and patients with bacterial overgrowth of the small intestine. J Clin Invest. 1977;59:1158-1166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 623] [Cited by in F6Publishing: 597] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 36. | Gupta A, Dhiman RK, Kumari S, Rana S, Agarwal R, Duseja A, Chawla Y. Role of small intestinal bacterial overgrowth and delayed gastrointestinal transit time in cirrhotic patients with minimal hepatic encephalopathy. J Hepatol. 2010;53:849-855. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 141] [Article Influence: 10.1] [Reference Citation Analysis (0)] |