Published online Mar 7, 2012. doi: 10.3748/wjg.v18.i9.930
Revised: September 9, 2011
Accepted: December 31, 2011
Published online: March 7, 2012
AIM: To investigate the effect of drinking sulphate-bicarbonate-calcium thermal water (TW) on risk factors for atherosclerosis and cholesterol gallstone disease.
METHODS: Postmenopausal women with functional dyspepsia and/or constipation underwent a 12 d cycle of thermal (n = 20) or tap (n = 20) water controlled drinking. Gallbladder fasting volume at ultrasound, blood vitamin E, oxysterols (7-β-hydroxycholesterol and 7-ketocholesterol), bile acid (BA), triglycerides, total/low density lipoprotein and high density lipoprotein cholesterol were measured at baseline and at the end of the study. Food consumption, stool frequency and body weight were recorded daily.
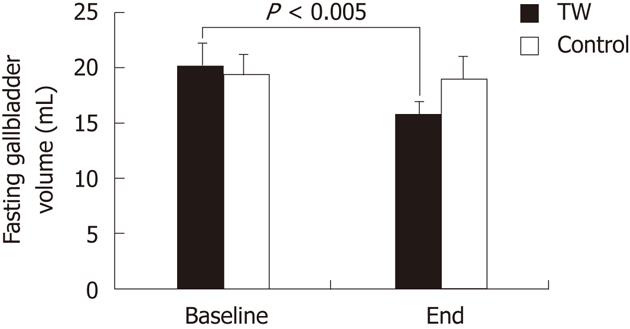
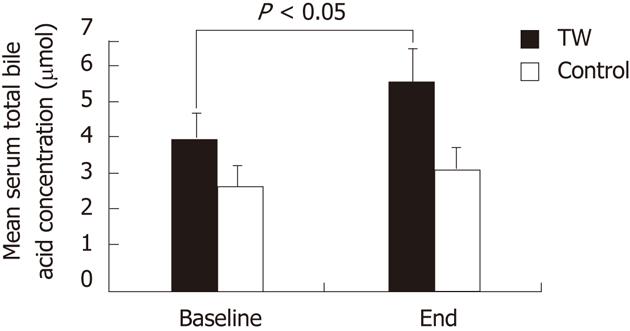
RESULTS: Blood lipids, oxysterols and vitamin E were not affected by either thermal or tap water consumption. Fasting gallbladder volume was significantly (P < 0.005) smaller at the end of the study than at baseline in the TW (15.7 ± 1.1 mL vs 20.1 ± 1.7 mL) but not in the tap water group (19.0 ± 1.4 mL vs 19.4 ± 1.5 mL). Total serum BA concentration was significantly (P < 0.05) higher at the end of the study than at baseline in the TW (5.83 ± 1.24 μmol vs 4.25 ± 1.00 μmol) but not in the tap water group (3.41 ± 0.46 μmol vs 2.91 ± 0.56 μmol). The increased BA concentration after TW consumption was mainly accounted for by glycochenodeoxycholic acid. The number of pasta (P < 0.001), meat (P < 0.001) and vegetable (P < 0.005) portions consumed during the study and of bowel movements per day (P < 0.05) were significantly higher in the TW than in the tap water group. Body weight did not change at the end of the study as compared to baseline in both groups.
CONCLUSION: Sulphate-bicarbonate-calcium water consumption has a positive effect on lithogenic risk and intestinal transit and allows maintenance of a stable body weight despite a high food intake.
- Citation: Corradini SG, Ferri F, Mordenti M, Iuliano L, Siciliano M, Burza MA, Sordi B, Caciotti B, Pacini M, Poli E, Santis AD, Roda A, Colliva C, Simoni P, Attili AF. Beneficial effect of sulphate-bicarbonate-calcium water on gallstone risk and weight control. World J Gastroenterol 2012; 18(9): 930-937
- URL: https://www.wjgnet.com/1007-9327/full/v18/i9/930.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i9.930
Atherosclerosis, colelithiasis and obesity are very frequent and interrelated diseases among postmenopausal women[1-4].
High serum triglycerides and total/low-density lipoprotein (LDL) cholesterol are important risk factors for atherosclerosis, heart attack and stroke[5]. Also oxidative stress, particularly the oxidation of LDL, plays a key role in atherogenesis due to the production of reactive oxygen species. New markers based on the detection of lipid peroxidation products by mass spectroscopy, such as oxysterols including 7-β-hydroxycholesterol and 7-ketocholesterol, are specific, sensitive and reliable markers of systemic oxidative stress in vivo[6-8]. In addition, oxysterol coupled to vitamin E measurement in plasma can be used for estimating systemic oxidant stress/antioxidant balance[8].
Cholesterol gallstone disease is very common in postmenopausal women, with incidence ranging from 22% to 30% in Western countries, and this disorder is one of the most common and costly of all digestive diseases[3]. Cholesterol gallstone pathogenesis is complex and multifactorial, involving genetic defects and environmental factors[9-11]. Changes in bile acid (BA), cholesterol and triglyceride metabolism, gallbladder reduced function and prolonged colonic transit time are critical factors in the pathogenesis of gallstones[12-15].
Obesity and overweight are risk factors for both atherosclerosis and gallstone disease[16]. Some data suggest that body weight reduction can be achieved by acceleration of intestinal transit in humans and by BA feeding in animals[17-21].
Thermal water (TW) consumption has been shown to ameliorate blood cholesterol patterns and systemic oxidative stress, and reduce oro-fecal transit time and gallbladder fasting volume[22-26]. No data are available on the effect of TW on BA pool composition and plasma oxysterols.
In the present study, we investigated in postmenopausal women the effect of drinking sulphate-bicarbonate-calcium TW on blood cholesterol, triglycerides, oxysterols, vitamin E and BA, and on gallbladder fasting volume, intestinal transit rate and body weight.
The study protocol was approved by the Ethics Committee of the University of Rome Sapienza and informed written consent was obtained from all patients.
Forty postmenopausal (at least 1 year) women with functional dyspepsia and/or constipation participated in this study. Patients were divided into 2 groups: (1) TW group were 20 patients enrolled by the medical staff of Chianciano thermal centre (Tuscany, Italy); (2) control (CTRL) group were 20 patients enrolled by the medical staff of the Gastroenterology Division of Department of Clinical Medicine at the Sapienza University (Rome, Italy). Diagnosis of functional dyspepsia and/or constipation was made based on the Roma III criteria[27,28].
Exclusion criteria were a history of liver, pancreatic, gallbladder (including sonographic evidence of gallstones) or other gastrointestinal diseases, lipid disorders, diabetes, severe high blood pressure (diastolic > 110 mmHg, systolic > 180 mmHg), cancer, surgical resection, and thyroid, neurological, muscular, rheumatological and immunological diseases. Patients were also excluded if they were heavy drinkers, heavy smokers or habitual drinkers of more than 3 cups of espresso coffee every day. Individuals enrolled were not receiving estrogen replacement therapy or any medication known to affect lipid metabolism, and were not taking vitamin, mineral, or phytoestrogen supplements. Participants had not consumed diets intended to cause weight loss within 1 year of selection.
Between 8 and 9 a.m. on days 1 and 13 after an overnight fast and before drinking water, all enrolled patients underwent blood sampling and abdominal ultrasonography, and they had daily body weight measurements, according to international standards, using a digital scale that was calibrated, having a capacity of up to 150 kg[29].
Patients in the TW group underwent a 12 d cycle of TW treatment by drinking 500 mL of “Acqua Santa of Chianciano Terme” sulphate-bicarbonate-calcium water, at a temperature of 33 °C, every day in the morning in the fasted state, over a 30 min period. The control group drank Rome tap water at a temperature of 10-12 °C using the same schedule. The chemical composition of the “Acqua Santa of Chianciano Terme” sulphate-bicarbonate-calcium water and of the Rome tap water is reported in Table 1. Each day of the study all patients filled a stool diary[30] and a food and beverage frequency daily diary which asked for the number of portions consumed for the following items: Pasta, pizza, meat, fish, vegetables, bread, desserts, soft drinks, fruits, milk, dairy products, legumes and espresso coffee.
| Thermal water (TW) | Tap water (CTRL) | |
| pH | 6.8 | 7.5 |
| Fixed residue at 180 °C (mg/L) | 3280 | 390 |
| Sulphate (mg/L) | 1840 | 15 |
| Bicarbonate (mg/L) | 730 | - |
| Calcium (mg/L) | 840 | 98 |
| Magnesium (mg/L) | 180 | 19 |
| Sodium (mg/L) | 41 | 5.5 |
| Chloride (mg/L) | 29.4 | 6.5 |
| Potassium (mg/L) | 7 | 3 |
| Fluoride (mg/L) | 2 | 0.2 |
| Bromide (mg/L) | 0.2 | - |
| Carbon dioxide (cc/L) | 537 | - |
| Strontium (mg/L) | 0.1 | - |
| Iron (µg/L) | 0.8 | 5 |
| Manganese (μg/L) | - | 0.3 |
| Nitrate (mg/L) | - | 3.8 |
Fasting gallbladder volume was calculated by using the ellipsoid formula on the average of 2 sonographical gallbladder measurements[31].
Plasma and serum were stored at -80 °C. Plasma levels of α-tocopherol were analyzed by high performance liquid chromatography (HPLC), and 7-β-hydroxycholesterol and 7-ketocholesterol were measured from the same sample by mass spectrometry using an isotope dilution method[8]. Serum triglycerides, total and high-density lipoprotein (HDL) cholesterol were measured by a colorimetric method. LDL cholesterol was calculated according to the Friedewald Formula[32].
BA standards were obtained from Sigma Aldrich (St. Louis, United States). Total serum BA concentration was determined enzymatically by the 3α-hydroxysteroid-dehydrogenase assay (Stereognost 3a, Pho, Nycomed, AS, Torsov, Norway). The qualitative and quantitative BA composition was assessed by an HPLC-electrospray-mass spectrometry method, as previously reported with a slight modification[33]. Isolute C18 cartridges were obtained from International Sorbent Technology LTD (Hengoed, United Kingdom). The solid phase extraction cartridge was conditioned with 5 mL of methyl alcohol and 5 mL of water prior to the sample loading. Serum samples were diluted 1:6 (v/v) with 0.1 N solution of NaOH and heated to 64 °C for 30 min. Afterwards, the serum sample was loaded on the conditioned cartridge and then washed with 10 mL of water. The cartridge was then eluted with 5 mL of methyl alcohol. The eluate was dried under vacuum and then reconstituted with the mobile phase (70:30 v/v ammonium acetate buffer/acetonitrile) and injected into the HPLC-electrospray-mass spectrometry instrument. The recovery of all BAs ranged from 80% to 96%. The chromatographic system consisted of a Waters Alliance 2695 HPLC system. The separation was obtained using a 150 mm × 2.00 mm, 4 μm Phenomenex Sinergy Hydro-RP C18 column with a mobile phase consisting of 15 mmol ammonium acetate buffer (pH 5)/acetonitrile. The mobile phase was delivered at a flow rate of 0.150 mL/min, with a total HPLC-electrospray-mass spectrometry run time of 30 min. Mass spectra were obtained with a Quattro LC mass spectrometer (Micromass, United Kingdom) equipped with electrospray source. All BA ions were monitored in a negative mode by the Multiple Reaction Monitoring mode. A seven point calibration curve, ranging from blank to 10 μmol was prepared by spiking BA-free serum with the analytes for serum analysis. Quantification of the analytes in the sample was performed on the peak area, by external calibration. The inter-assay precision and accuracy were determined by analyzing three calibration curves with quality control samples at one-concentration level (1 μmol) on 2 d. The value for the coefficient of variation (%) near the limit of detection was 1%-2%.
Analysis of data was carried out using the “Statistical Package for Social Sciences (SPSS) for Windows (SPSS version 17.0, Chicago, IL, United States). Data are reported as mean ± SE. Intergroup differences between categorical variables were estimated by the χ2 test. The non-parametric Kolmogorov-Smirnov test was used to verify the normal distribution of the continuous variables data set. When the data set was normally distributed, the Student t test for coupled or uncoupled data was used as appropriate. When the data set was not normally distributed, the variables were analyzed by the Mann-Whitney U-test and by the Wilcoxon test as appropriate. A significant level of 0.05 (P < 0.05) was chosen to assess the statistical significance.
No intergroup difference was found in terms of age, weight, height, body mass index (BMI) and diagnosis (Table 2). Eight patients in each group had constipation either alone or associated with dyspepsia.
| TW | CTRL | P value | |
| Age (yr) | 64.0 ± 1.4 | 61.2 ± 1.8 | NS |
| Weight (kg) | 64.4 ± 2.3 | 61.1 ± 1.5 | NS |
| Height (cm) | 161 ± 0.01 | 160 ± 0.01 | NS |
| BMI (kg/m2) | 24.9 ± 0.9 | 24.0 ± 0.6 | NS |
| Diagnosis n (%) | |||
| Dyspepsia only | 12 (60) | 12 (60) | NS |
| Constipation only | 6 (30) | 5 (25) | NS |
| Constipation + dyspepsia | 2 (10) | 3 (15) | NS |
As shown in Table 3, we did not find any intergroup (TW vs CTRL) difference in serum levels of total, HDL and LDL cholesterol and triglycerides. Plasma 7-β-hydroxycholesterol, 7-ketocholesterol, α-tocopherol, γ-tocopherol and oxysterol to tocopherol ratio, both at baseline and at the end of the study, did not differ between the TW and the CTRL group. In addition, no change in blood lipids or oxidative stress was found at the end of treatment with respect to baseline when each group was considered separately.
| TW | CTRL | |||
| Baseline | End | Baseline | End | |
| Total cholesterol (mg/dL) | 178.7 ± 5.8 | 182.4 ± 6.3 | 181.5 ± 7.6 | 177.4 ± 6.5 |
| HDL cholesterol (mg/dL) | 62.3 ± 4.7 | 63.7 ± 4.7 | 56.7 ± 5.0 | 59.4 ± 6.1 |
| LDL cholesterol (mg/dL) | 100.4 ± 8.0 | 101.9 ± 8.7 | 103.6 ± 8.5 | 93.9 ± 7.7 |
| Triglycerides (mg/dL) | 79.9 ± 7.5 | 84.0 ± 10.2 | 106.1 ± 11.4 | 120.7 ± 17.9 |
| 7β-HC (ng/mL) | 51.4 ± 12.3 | 70.5 ± 15.1 | 57.0 ± 8.2 | 45.5 ± 8.9 |
| 7-KC (ng/mL) | 93.7 ± 32.5 | 103.8 ± 29.1 | 38.6 ± 8.4 | 53.1 ± 25.9 |
| 7β-HC + 7-KC (ng/mL) | 145.1 ± 44.7 | 174.3 ± 43.6 | 95.7 ± 16.2 | 98.6 ± 34.5 |
| α-TCP (mg/dL) | 1.46 ± 0.1 | 1.43 ± 0.1 | 1.40 ± 0.1 | 1.43 ± 0.1 |
| γ-TCP (mg/dL) | 0.74 ± 0.1 | 0.75 ± 0.1 | 0.67 ± 0.05 | 0.79 ± 0.1 |
| α-TCP + γ-TCP (mg/dL) | 2.20 ± 0.1 | 2.18 ± 0.1 | 2.07 ± 0.1 | 2.22 ± 0.1 |
| 7β-HC + 7-KC/α-TCP + γ-TCP | 71.1 ± 22.6 | 65.7 ± 14.3 | 52.5 ± 9.2 | 54.1 ± 21.9 |
As shown in Figure 1, the mean fasting gallbladder volume did not differ between the TW and the CTRL group both at baseline and at the end of the study. Fasting gallbladder volume was significantly (P < 0.005) smaller at the end of the study than at baseline in the TW (15.7 ± 1.1 mL vs 20.1 ± 1.7 mL) but not in the CTRL group (19.0 ± 1.4 mL vs 19.4 ± 1.5 mL).
As shown in Figure 2, although there was a trend for higher baseline values in the TW than in the CTRL group, the mean serum total BA concentration did not significantly differ between the TW and the CTRL group both at baseline and at the end of the study. Serum total BA concentration was significantly (P < 0.05) higher at the end of the study than at baseline in the TW (5.83 ± 1.24 μmol vs 4.25 ± 1.00 μmol) but not in the CTRL group (3.41 ± 0.46 μmol vs 2.91 ± 0.56 μmol).
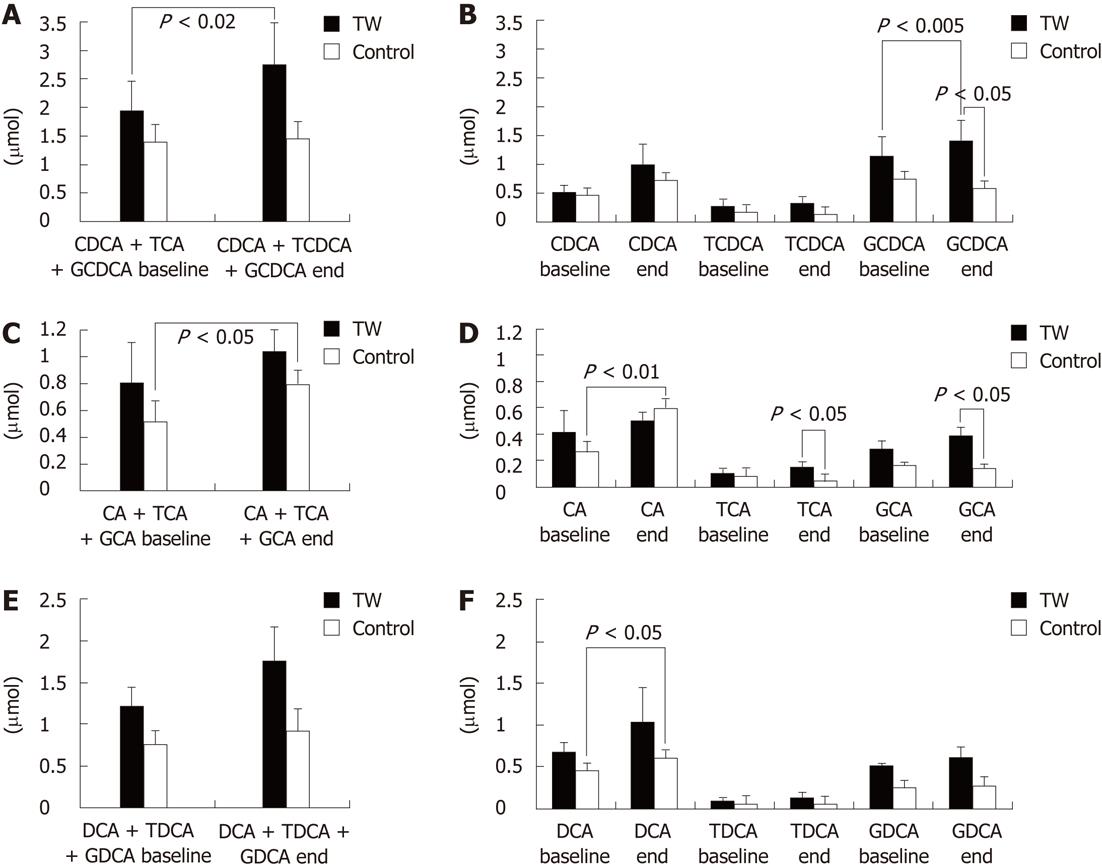
With regard to the serum BA molecular species, as shown in Figure 3, no intergroup difference was found at baseline. At the end of the study however, the TW as compared to the CTRL group had significantly (P < 0.05) higher glycochenodeoxycholic acid (GCDCA) (1.41 ± 0.35 μmol vs 0.59 ± 0.07 μmol, respectively), taurocholic acid (TCA) (0.15 ± 0.04 μmol vs 0.05 ± 0.02 μmol, respectively) and glycocholic acid (GCA) (0.39 ± 0.10 μmol vs 0.14 ± 0.02 μmol, respectively). No other intergroup difference was found at the end of the study.
When the BA molecular species serum concentrations were compared separately in each study group at the end of the study with regard to baseline, in the TW group the mean GCDCA concentration at the end of the study was significantly higher (P < 0.005) than at baseline (1.41 ± 0.85 μmol vs 1.15 ± 0.35 μmol). On the contrary, in the CTRL group there was a trend (P = 0.062) for a lower GCDCA concentration at the end with respect to baseline (0.59 ± 0.07 μmol vs 0.75 ± 0.21 μmol). The mean free cholic acid (CA) concentration was significantly (P < 0.01) higher at the end of the study than at baseline in the CTRL (0.60 ± 0.14 μmol vs 0.27 ± 0.09 μmol) but not in the TW group (0.50 ± 0.12 μmol vs 0.42 ± 0.18 μmol). The mean free deoxycholic acid (DCA) concentration was significantly (P < 0.05) higher at the end of the study than at baseline in the CTRL (0.60 ± 0.16 μmol vs 0.45 ± 0.13 μmol) but not in the TW group (1.03 ± 0.44 μmol vs 0.67 ± 0.16 μmol). The other BA molecular species did not change at the end as compared to baseline when each group was considered separately. The sum of free chenodeoxycholic acid (CDCA), GCDCA and taurochenodeoxycholic acid was significantly (P < 0.02) higher at the end of the study than at baseline in the TW (2.75 ± 0.70 μmol vs 1.95 ± 0.58 μmol) but not in the CTRL group (1.46 ± 0.20 μmol vs 1.40 ± 0.35 μmol). The sum of CA, GCA and TCA was significantly (P < 0.05) higher at the end of the study than at baseline in the CTRL (0.80 ± 0.14 μmol vs 0.52 ± 0.11 μmol) but not in the TW group (1.04 ± 0.20 μmol vs 0.81 ± 0.27 μmol).
As shown in Table 4, the number of pasta (P < 0.001), meat (P < 0.001) and vegetable (P < 0.005) portions consumed during the study period was significantly higher by approximately two-fold in the TW than in the CTRL group, while bread consumption was significantly (P < 0.05) less frequent, being half the amount in the TW than in the CTRL group. No intergroup difference was found with regard to pizza, dessert, soft drink, fruit, fish, milk, dairy product, legume and coffee espresso consumption.
| TW | Control | P value | |
| Pasta | 16.40 ± 1.22 | 8.80 ± 1.05 | < 0.001 |
| Bread | 4.32 ± 1.40 | 8.90 ± 1.57 | 0.01 |
| Pizza | 0.58 ± 0.23 | 1.01 ± 0.31 | NS |
| Sweets | 9.57 ± 1.87 | 7.08 ± 1.02 | NS |
| Soft drinks | 4.82 ± 1.40 | 4.30 ± 1.59 | NS |
| Fruits | 17.60 ± 1.69 | 14.59 ± 2.24 | NS |
| Meat | 11.52 ± 1.00 | 5.90 ± 0.51 | < 0.001 |
| Fish | 4.05 ± 0.75 | 3.20 ± 0.38 | NS |
| Milk | 6.00 ± 1.21 | 6.89 ± 0.87 | NS |
| Dairy products | 6.32 ± 1.23 | 4.04 ± 0.50 | NS |
| Legumes | 0.98 ± 0.36 | 0.84 ± 0.23 | NS |
| Vegetables | 22.80 ± 2.40 | 13.51 ± 1.08 | 0.002 |
| Coffee | 14.08 ± 2.31 | 13.32 ± 2.41 | NS |
During the study period, the TW group had a significantly (P < 0.05) higher number of bowel movements per day than the CTRL group (1.077 ± 0.057 vs 0.893 ± 0.055, respectively). Body weight did not differ between the TW and the CTRL group both at baseline (64.4 ± 2.4 kg vs 61.1 ± 1.5 kg, respectively) and at the end of the study (64.3 ± 2.4 kg vs 61.3 ± 1.4 kg, respectively). In addition, no change in body weight was found at the end of the study with respect to baseline when each group was considered separately.
The main finding of the present study is that 12 d of sulphate-bicarbonate-calcium TW, but not tap water, administration to gallstone-free postmenopausal women with functional dyspepsia and/or constipation is associated with a reduction of fasting gallbladder volume and an increase in fasting serum BA concentration, especially GCDCA. The effects of sulphate-bicarbonate-calcium TW administration on fasting gallbladder volume and serum BA that we found can be considered protective from gallstone development. In fact, a relatively high fasting gallbladder volume, indicative of a gallbladder motility defect, has been shown to be associated with gallstones. Conversely, a beneficial effect of preserved gallbladder motility on gallstone recurrence has been demonstrated after extracorporeal shock-wave lithotripsy[14,34-36].
Since CDCA molar percent in serum has been shown to correlate with that in gallbladder bile, the increased concentration of serum GCDCA (the major form of CDCA in humans) that we found after sulphate-bicarbonate-calcium TW administration is likely to reflect bile enrichment with this BA[37,38]. Although a direct measurement of the qualitative and quantitative BA composition in bile is the best predictor of gallstone risk, our findings in serum suggest that sulphate-bicarbonate-calcium TW administration can be considered protective from gallstone development. In fact, it has been shown that cholesterol gallstone patients have a lower CDCA and a higher DCA content in gallbladder bile than gallstone-free controls[39]. In addition, pharmacological CDCA administration has been used as a litholytic/preventive treatment against gallstones and ameliorates cholesterol solubility in BA[40].
The beneficial effect of sulphate-bicarbonate-calcium TW administration on gallbladder motility has been already demonstrated, but the underlying mechanisms are not clear[41]. The effect of sulphate-bicarbonate-calcium TW consumption on the BA pool has never been shown previously and our present data do not allow the clarification of the mechanisms. In fact, limitations of the present study are the lack of measurements of intestinal transit time and of BA hepatic synthesis and fecal losses. However, as indirectly suggested by the higher frequency of bowel movements that we found in the TW than in the CTRL group, it can be hypothesized that TW consumption accelerates intestinal transit. This change in intestinal transit is in agreement with the increased fecal scour score described in pigs ingesting a high mineral sulphated water[42] and should be secondary to an osmotic mechanism, although the warm temperature of the TW could also play a role[41]. The acceleration of intestinal transit, as well as the improved gallbladder motility, are likely to increase the frequency of BA enterohepatic circulation and fecal losses with a secondary stimulation of primary BA (especially CDCA) hepatic synthesis. The increased frequency of BA enterohepatic circulation and the enrichment of the BA pool with CDCA could then further accelerate colonic transit. The latter hypothesis is in agreement with previously published data showing: (1) a positive correlation between the rate of BA synthesis and colonic transit (the higher the synthesis the faster colonic transit)[43]; (2) a positive correlation between serum CDCA and intestinal transit (the higher the concentration the faster intestinal transit)[14]; and (3) that CDCA administration accelerates colonic transit in healthy volunteers and in female patients with constipation-predominant irritable bowel syndrome[43,44]. The second finding of the present study is that body weight and blood total, HDL and LDL cholesterol, triglycerides, oxysterols and vitamin E were not affected by 12 d of either sulphate-bicarbonate-calcium or tap water consumption. Interestingly, we found that the TW, as compared to the CTRL group, showed a doubling of frequency of pasta, meat and vegetable consumption during the study period suggesting that drinking sulphate-bicarbonate-calcium TW allows maintenance of stable body weight and blood cardiovascular risk factors under conditions of overfeeding. Again, our present study does not allow clarification of the mechanisms for this unexpected finding. In fact, other than the lack of characterization of BA enterohepatic circulation, we did not assess gastric emptying and energy expenditure. However, the increased food intake in our TW group could be explained by an increased gastrointestinal emptying and more frequent BA enterohepatic circulation, with GCDCA enrichment. In agreement with this hypothesis, both the ingestion of a high mineral sulphated water in pigs[42] and the administration of CDCA in humans have been shown to increase food consumption. Furthermore, the increased serum BA concentration that we found during sulphate-bicarbonate-calcium TW consumption could directly avert weight gain, despite increased food consumption. In fact, serum BAs have been recognized as important modulators of whole-body metabolism, by increasing energy expenditure in brown adipose tissue and in muscles, through promotion of intracellular thyroid hormone activation secondary to the activation of the TGR5-signaling pathway[21].
In conclusion, sulphate-bicarbonate-calcium TW consumption in postmenopausal women with functional dyspepsia and/or constipation has a positive effect on the lithogenic risk and intestinal transit and allows maintenance of a stable body weight despite a high food intake. Further studies are needed to confirm these effects of TW in asymptomatic subjects and to prove its potential benefit in weight loss treatments.
Atherosclerosis, gallstones and obesity are very frequent and interrelated diseases, with a very high socioeconomic impact worldwide. High triglycerides, total/low-density lipoprotein cholesterol and increased oxidative stress in blood are important risk factors for atherosclerosis and cardiovascular diseases. In addition to cholesterol and triglyceride metabolism, bile acid (BA) metabolism, gallbladder motility and intestinal motility are critical factors in the pathogenesis of gallstones. Obesity and overweight are risk factors for both atherosclerosis and gallstone disease.
Thermal water (TW), and especially sulphate-bicarbonate mineral waters, are used to treat several biliary and digestive tract diseases. In the present study, The authors investigated the effect of drinking sulphate-bicarbonate-calcium TW on risk factors for: (1) atherosclerosis (i.e., cholesterol, triglycerides and markers of oxidative stress in blood); (2) gallstones (i.e., BAs in blood, gallbladder and intestinal motility; and (3) diet and body weight.
TW drinking has been shown to ameliorate intestinal and gallbladder motility and blood cholesterol and oxidative stress markers. However, in previous studies oxidative stress was evaluated by using methods with poor physiological significance in vivo. No data are available on the effect of TW on BA metabolism and body weight. In the present study, for the first time we investigated the effect of drinking sulphate-bicarbonate-calcium TW on BA metabolism and body weight. In addition, the authors evaluated the effect of drinking sulphate-bicarbonate-calcium TW on oxidative stress by measuring sensitive and specific markers of enhanced oxidant stress in vivo, such as oxysterols, or antioxidant defense by measuring α-tocopherol.
The results suggest that sulphate-bicarbonate-calcium water consumption has a positive effect on the risk of gallstone development and allows maintenance of a stable atherosclerosis risk and body weight despite a high food intake. This study might be useful in preparation of preventive strategies for atherosclerosis and gallstones in overweight and obese subjects.
Oxysterols are oxidation products of cholesterol, and among them 7-β-hydroxycholesterol and 7-ketocholesterol are produced nonenzymatically via a free radical-mediated mechanism and, thus, are very good markers of oxidant stress in vivo. BAs are steroid acids found predominantly in the bile and, in lower concentrations, in serum of mammals. Besides their well-established roles in lipid absorption and homeostasis and cholesterol biliary solubilization, BAs also act as metabolically active signaling molecules.
The study is of particular interest to those involved in practical medicine. The authors’ data might be used for the prevention of atherosclerosis development and gallstone disease in postmenopausal women and probably for the treatment of these diseases.
Peer reviewer: Vasiliy I Reshetnyak, MD, PhD, Professor, Scientist Secretary of the Scientific Research Institute of General Reanimatology, 25-2, Petrovka str., 107031 Moscow, Russia
S- Editor Gou SX L- Editor Logan S E- Editor Xiong L
| 1. | Sutton-Tyrrell K, Lassila HC, Meilahn E, Bunker C, Matthews KA, Kuller LH. Carotid atherosclerosis in premenopausal and postmenopausal women and its association with risk factors measured after menopause. Stroke. 1998;29:1116-1121. [PubMed] [Cited in This Article: ] |
| 2. | Attili AF, Capocaccia R, Carulli N, Festi D, Roda E, Barbara L, Capocaccia L, Menotti A, Okolicsanyi L, Ricci G. Factors associated with gallstone disease in the MICOL experience. Multicenter Italian Study on Epidemiology of Cholelithiasis. Hepatology. 1997;26:809-818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 131] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Novacek G. Gender and gallstone disease. Wien Med Wochenschr. 2006;156:527-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Pi-Sunyer FX. The obesity epidemic: pathophysiology and consequences of obesity. Obes Res. 2002;10 Suppl 2:97S-104S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 501] [Cited by in F6Publishing: 477] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 5. | Schultheis AH. Hypercholesterolemia: prevention, detection and management. Nurse Pract. 1990;15:40-46, 51-55-56. [PubMed] [Cited in This Article: ] |
| 6. | Brown AJ, Jessup W. Oxysterols and atherosclerosis. Atherosclerosis. 1999;142:1-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 685] [Cited by in F6Publishing: 687] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 7. | Björkhem I, Diczfalusy U. Oxysterols: friends, foes, or just fellow passengers? Arterioscler Thromb Vasc Biol. 2002;22:734-742. [PubMed] [Cited in This Article: ] |
| 8. | Iuliano L, Micheletta F, Natoli S, Ginanni Corradini S, Iappelli M, Elisei W, Giovannelli L, Violi F, Diczfalusy U. Measurement of oxysterols and alpha-tocopherol in plasma and tissue samples as indices of oxidant stress status. Anal Biochem. 2003;312:217-223. [PubMed] [Cited in This Article: ] |
| 9. | Khanuja B, Cheah YC, Hunt M, Nishina PM, Wang DQ, Chen HW, Billheimer JT, Carey MC, Paigen B. Lith1, a major gene affecting cholesterol gallstone formation among inbred strains of mice. Proc Natl Acad Sci USA. 1995;92:7729-7733. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 158] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Apstein MD, Carey MC. Pathogenesis of cholesterol gallstones: a parsimonious hypothesis. Eur J Clin Invest. 1996;26:343-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 86] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Marschall HU, Katsika D, Rudling M, Einarsson C. The genetic background of gallstone formation: an update. Biochem Biophys Res Commun. 2010;396:58-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Smelt AH. Triglycerides and gallstone formation. Clin Chim Acta. 2010;411:1625-1631. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Stender S, Frikke-Schmidt R, Nordestgaard BG, Tybjaerg-Hansen A. Sterol transporter adenosine triphosphate-binding cassette transporter G8, gallstones, and biliary cancer in 62,000 individuals from the general population. Hepatology. 2011;53:640-648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Colecchia A, Mazzella G, Sandri L, Azzaroli F, Magliuolo M, Simoni P, Bacchi-Reggiani ML, Roda E, Festi D. Ursodeoxycholic acid improves gastrointestinal motility defects in gallstone patients. World J Gastroenterol. 2006;12:5336-5343. [PubMed] [Cited in This Article: ] |
| 15. | Fan Y, Wu SD, Fu BB. Effect of intestinal transit on the formation of cholesterol gallstones in hamsters. Hepatobiliary Pancreat Dis Int. 2007;6:513-515. [PubMed] [Cited in This Article: ] |
| 16. | Bortnichak EA, Freeman DH, Ostfeld AM, Castelli WP, Kannel WB, Feinleib M, McNamara PM. The association between cholesterol cholelithiasis and coronary heart disease in Framingham, Massachusetts. Am J Epidemiol. 1985;121:19-30. [PubMed] [Cited in This Article: ] |
| 17. | Miller LJ, Gorman CA, Go VL. Gut-thyroid interrelationships. Gastroenterology. 1978;75:901-911. [PubMed] [Cited in This Article: ] |
| 18. | Holgate AM, Read NW. Relationship between small bowel transit time and absorption of a solid meal. Influence of metoclopramide, magnesium sulfate, and lactulose. Dig Dis Sci. 1983;28:812-819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 103] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Read NW. Diarrhée motrice. Clin Gastroenterol. 1986;15:657-686. [PubMed] [Cited in This Article: ] |
| 20. | Bortolotti M, Levorato M, Lugli A, Mazzero G. Effect of a balanced mixture of dietary fibers on gastric emptying, intestinal transit and body weight. Ann Nutr Metab. 2008;52:221-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484-489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1641] [Cited by in F6Publishing: 1618] [Article Influence: 89.9] [Reference Citation Analysis (0)] |
| 22. | Schoppen S, Pérez-Granados AM, Carbajal A, Oubiña P, Sánchez-Muniz FJ, Gómez-Gerique JA, Vaquero MP. A sodium-rich carbonated mineral water reduces cardiovascular risk in postmenopausal women. J Nutr. 2004;134:1058-1063. [PubMed] [Cited in This Article: ] |
| 23. | Capurso A, Solfrizzi V, Panza F, Mastroianni F, Torres F, Del Parigi A, Colacicco AM, Capurso C, Nicoletti G, Veneziani B. Increased bile acid excretion and reduction of serum cholesterol after crenotherapy with salt-rich mineral water. Aging (Milano). 1999;11:273-276. [PubMed] [Cited in This Article: ] |
| 24. | Benedetti S, Benvenuti F, Nappi G, Fortunati NA, Marino L, Aureli T, De Luca S, Pagliarani S, Canestrari F. Antioxidative effects of sulfurous mineral water: protection against lipid and protein oxidation. Eur J Clin Nutr. 2009;63:106-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Costantino M, Giuberti G, Caraglia M, Lombardi A, Misso G, Abbruzzese A, Ciani F, Lampa E. Possible antioxidant role of SPA therapy with chlorine-sulphur-bicarbonate mineral water. Amino Acids. 2009;36:161-165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Gasbarrini G, Candelli M, Graziosetto RG, Coccheri S, Di Iorio F, Nappi G. Evaluation of TW in patients with functional dyspepsia and irritable bowel syndrome accompanying constipation. World J Gastroenterol. 2006;12:2556-2562. [PubMed] [Cited in This Article: ] |
| 27. | Tack J, Talley NJ, Camilleri M, Holtmann G, Hu P, Malagelada JR, Stanghellini V. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466-1479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1243] [Cited by in F6Publishing: 1173] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 28. | Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480-1491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3413] [Cited by in F6Publishing: 3321] [Article Influence: 184.5] [Reference Citation Analysis (1)] |
| 29. | Lohman TG, Roche AF, Martorell R. Stature, recumbent length, weight. Champaign, IL: Human Kinetics Books 1991; 3–8. [Cited in This Article: ] |
| 30. | Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920-924. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1858] [Cited by in F6Publishing: 1988] [Article Influence: 73.6] [Reference Citation Analysis (0)] |
| 31. | Dodds WJ, Groh WJ, Darweesh RM, Lawson TL, Kishk SM, Kern MK. Sonographic measurement of gallbladder volume. AJR Am J Roentgenol. 1985;145:1009-1011. [PubMed] [Cited in This Article: ] |
| 32. | Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499-502. [PubMed] [Cited in This Article: ] |
| 33. | Roda A, Gioacchini AM, Cerrè C, Baraldini M. High-performance liquid chromatographic-electrospray mass spectrometric analysis of bile acids in biological fluids. J Chromatogr B Biomed Appl. 1995;665:281-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 53] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Pauletzki J, Althaus R, Holl J, Sackmann M, Paumgartner G. Gallbladder emptying and gallstone formation: a prospective study on gallstone recurrence. Gastroenterology. 1996;111:765-771. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 64] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Portincasa P, van Erpecum KJ, van De Meeberg PC, Dallinga-Thie GM, de Bruin TW, van Berge-Henegouwen GP. Apolipoprotein E4 genotype and gallbladder motility influence speed of gallstone clearance and risk of recurrence after extracorporeal shock-wave lithotripsy. Hepatology. 1996;24:580-587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Venneman NG, vanBerge-Henegouwen GP, Portincasa P, Stolk MF, Vos A, Plaisier PW, van Erpecum KJ. Absence of apolipoprotein E4 genotype, good gallbladder motility and presence of solitary stones delay rather than prevent gallstone recurrence after extracorporeal shock wave lithotripsy. J Hepatol. 2001;35:10-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Whiting MJ, Watts JM. Prediction of the bile acid composition of bile from serum bile acid analysis during gallstone dissolution therapy. Gastroenterology. 1980;78:220-225. [PubMed] [Cited in This Article: ] |
| 38. | Han TQ, Zhang SD, Tang WH, Jiang ZY. Bile acids in serum and bile of patients with cholesterol gallstone. World J Gastroenterol. 1998;4:82-84. [PubMed] [Cited in This Article: ] |
| 39. | Hirota I, Chijiiwa K, Noshiro H, Nakayama F. Effect of chenodeoxycholate and ursodeoxycholate on nucleation time in human gallbladder bile. Gastroenterology. 1992;102:1668-1674. [PubMed] [Cited in This Article: ] |
| 40. | Tudyka J, Kratzer W, Janowitz P, Mason R, Wechsler JG. Combined bile acid therapy is more effective on biliary lipids and dissolution rates than monotherapy after gallstone lithotripsy. Am J Gastroenterol. 1995;90:1942-1948. [PubMed] [Cited in This Article: ] |
| 41. | Fraioli A, Menunni G, Petraccia L, Fontana M, Nocchi S, Grassi M. Sulphate-bicarbonate mineral waters in the treatment of biliary and digestive tract diseases. Clin Ter. 2010;161:163-168. [PubMed] [Cited in This Article: ] |
| 42. | Maenz DD, Patience JF, Wolynetz MS. The influence of the mineral level in drinking water and the thermal environment on the performance and intestinal fluid flux of newly-weaned pigs. J Anim Sci. 1994;72:300-308. [PubMed] [Cited in This Article: ] |
| 43. | Rao AS, Wong BS, Camilleri M, Odunsi-Shiyanbade ST, McKinzie S, Ryks M, Burton D, Carlson P, Lamsam J, Singh R. Chenodeoxycholate in females with irritable bowel syndrome-constipation: a pharmacodynamic and pharmacogenetic analysis. Gastroenterology. 2010;139:1549-158, 1558.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 44. | Odunsi-Shiyanbade ST, Camilleri M, McKinzie S, Burton D, Carlson P, Busciglio IA, Lamsam J, Singh R, Zinsmeister AR. Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel function. Clin Gastroenterol Hepatol. 2010;8:159-165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |