Copyright
©2012 Baishideng Publishing Group Co.
World J Gastroenterol. Sep 14, 2012; 18(34): 4684-4692
Published online Sep 14, 2012. doi: 10.3748/wjg.v18.i34.4684
Published online Sep 14, 2012. doi: 10.3748/wjg.v18.i34.4684
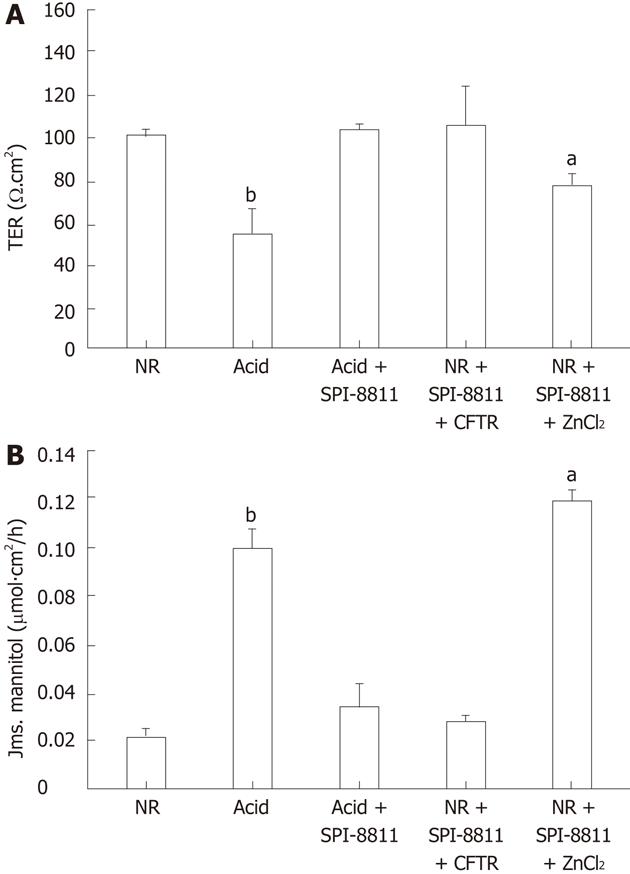
Figure 4 Electrical responses and 3H-mannitol fluxes of acid-injured porcine gastric mucosa.
A: Porcine gastric mucosa exposed to acid (pH 1.5 for 90 min) exhibited a significant drop in transepithelial electrical resistance (TER), which was completely blocked by pretreatment with SPI-8811. In attempt to discern which chloride channel was involved in the gastro protective mechanism of SPI-8811, ClC-2 and cystic fibrosis transmembrane conductance regulator (CFTR) were inhibited. Addition of the ClC-2 inhibitor ZnCl2 ameliorated the protective effect of SPI-8811 (TER, 100 Ω.cm2vs 80 Ω.cm2) whereas the CFTR inhibitor CFTR inhibitor 172 had no effect (TER, 100 Ω.cm2vs 100 Ω.cm2). Values represent mean ± SE, n = 6; aP < 0.05, bP < 0.01 vs all treatment groups; B: As an alternate measure of barrier permeability mucosal-to-serosal of 3H-mannitol flux were performed, and reveled a significant increase in mannitol permeability in acid injured tissues (bP < 0.01). Pretreatment with SPI-8811 blocked the increase in 3H-mannitol flux caused by acid injury, whereas blockade of ClC-2 inhibitor ZnCl2 block the protective effect of SPI-8811 (3H-mannitol flux, 0.04 μmol/L.cm2vs 0.12 μmol/L.cm2, aP < 0.05) on permeability but CFTR inhibitor had no effect on the level of protective properties of SPI-8811 on permeability (3H-mannitol flux, 0.04 μmol/L.cm2vs 0.05 μmol/L.cm2, bP < 0.01). Values represent mean ± SE, n = 6. NR: Normal Ringer’s.
- Citation: Nighot M, Moeser A, Ueno R, Blikslager A. Gastro protective properties of the novel prostone SPI-8811 against acid-injured porcine mucosa. World J Gastroenterol 2012; 18(34): 4684-4692
- URL: https://www.wjgnet.com/1007-9327/full/v18/i34/4684.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i34.4684