Published online Aug 21, 2012. doi: 10.3748/wjg.v18.i31.4145
Revised: May 8, 2012
Accepted: May 13, 2012
Published online: August 21, 2012
AIM: To compare the clinical outcome and pathologic features of non-alcoholic steatohepatitis (NASH) patients with hepatocellular carcinoma (HCC) and hepatitic C virus (HCV) patients with HCC (another group in which HCC is commonly seen) undergoing liver transplantation.
METHODS: Patients transplanted for HCV and NASH at our institution from January 2000 to April 2011 were analyzed. All explanted liver histology and pre-transplant liver biopsies were examined by two specialist liver histopathologists. Patient demographics, disease free survival, explant liver characteristics and HCC features (tumour number, cumulative tumour size, vascular invasion and differentiation) were compared between HCV and NASH liver transplant recipients.
RESULTS: A total of 102 patients with NASH and 283 patients with HCV were transplanted. The incidence of HCC in NASH transplant recipients was 16.7% (17/102). The incidence of HCC in HCV transplant recipients was 22.6% (64/283). Patients with NASH-HCC were statistically older than HCV-HCC patients (P < 0.001). A significantly higher proportion of HCV-HCC patients had vascular invasion (23.4% vs 6.4%, P = 0.002) and poorly differentiated HCC (4.7% vs 0%, P < 0.001) compared to the NASH-HCC group. A trend of poorer recurrence free survival at 5 years was seen in HCV-HCC patients compared to NASH-HCC who underwent a Liver transplantation (P = 0.11).
CONCLUSION: Patients transplanted for NASH-HCC appear to have less aggressive tumour features compared to those with HCV-HCC, which likely in part accounts for their improved recurrence free survival.
- Citation: Hernandez-Alejandro R, Croome KP, Drage M, Sela N, Parfitt J, Chandok N, Marotta P, Dale C, Wall W, Quan D. A comparison of survival and pathologic features of non-alcoholic steatohepatitis and hepatitis C virus patients with hepatocellular carcinoma. World J Gastroenterol 2012; 18(31): 4145-4149
- URL: https://www.wjgnet.com/1007-9327/full/v18/i31/4145.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i31.4145
The prevalence of obesity in North American Society continues to rise[1]. With this increasing rate of obesity there has been a concomitant increase in the prevalence of non-alcoholic fatty liver disease (NAFLD)[2]. The natural history of NAFLD is quite variable. It includes a spectrum ranging from reversible steatosis to steatohepatitis with hepatic fibrosis (NASH), and ultimately cirrhosis[3-5]. Up to 30% of adults in North America and Western Europe are known to have excess fat accumulation in the liver[6]. Of these, nearly 10% have NASH, which represents 2%-3% of all adults. There is speculation that NASH may soon become one of the main causes of End Stage Liver Disaese (ESLD) requiring liver transplantation in North America[7].
Hepatitis C virus (HCV) is one of the most common underlying liver diseases in hepatocellular carcinoma (HCC), accounting for about one-third of the cases of HCC in the United States[8]. It is well established that patients with NASH can progress to develop HCC with previous reports suggesting that the 5 year prevalence may be as high as 7.6%[9]. However as increasing numbers of HCC cases arising from NASH are being seen, it is important to clarify the outcomes and recurrence by comparing the clinical and pathological features of HCC due to NASH with those of HCC caused by one the more common underlying liver diseases in HCC, HCV infection, as a benchmark.
Previous studies have suggested that patients with NASH cirrhosis are less likely than those with HCV to get transplanted[10]. This may be in large part to a higher likelihood of being denied listing for co-morbid conditions. Previous authors have shown that NASH patients with diabetes, hypertension, body mass index (BMI) > 30 years and age > 60 years undergoing liver transplantation have a poor (50%) 1 year mortality[11]. However in appropriately selected NASH patients post liver transplant survival can fair at least as well as individuals who undergo transplant for other etiologies. The outcome of NASH patients with underlying HCC undergoing a liver transplantation compared to HCV patients with underlying HCC (another group in which HCC is commonly seen) has not been thoroughly investigated. Specifically the tumour characteristics in explanted livers and disease free survival between these groups have not been compared. The goal of the present study was to compare the clinical and pathological parameters as well as disease free survival in the two groups.
We performed a retrospective review on all patients who underwent liver transplantation (LT) for HCV or NASH cirrhosis from January 2000 to April 2011 at our institution. Patients less than 18 years of age were excluded. Data on these patients were prospectively entered in our transplant database. In order to confirm or refute the original histological diagnoses, all explanted liver histology and pre-transplant liver biopsies were re-examined by two specialist liver histopathologists who were blinded to the original diagnoses.
The etiology of the original liver disease was diagnosed by set criteria. NASH was determined to be the cause of chronic liver disease in patients with histological evidence of steatohepatitis in pre-transplant liver biopsies or in liver explants (steatosis, portal and/or lobular inflammation, hepatocyte ballooning, pericellular fibrosis and the presence of Mallory bodies)[12,13], in conjunction with no history of alcohol consumption. HCV-related liver disease was confirmed by explants pathology and the presence of HCV RNA.
All patients with a pre and post-transplant diagnosis of HCC were identified in both the NASH and HCV groups. Listed patients with known HCC all fell within Milan Criteria[14]. Patients who received pre-transplant radiofrequency ablation were excluded. HCC was confirmed histologically in the explanted liver. All Donation after Cardiac Death (DCD) organs were procured from controlled DCD donors using techniques previously published by our group[15]. Primary outcomes were patient survival as well as pathologic features of HCC (tumour number, cumulative tumour size, vascular invasion and differentiation). Level of differentiation of HCC tumours was graded using the Modified Edmondson-Steiner grading system[16]. Additional variables investigated included age at diagnosis, gender and α-feto-protein (AFP) levels. Recurrence free survival was taken at the time point of maximal follow-up.
All data are presented as means ± SD. Differences between groups were analyzed using the unpaired t test for continuous variables and by the χ2 test or continuity correction method for categorical variables. Survival curves for patient and graft survival were generated using the Kaplan-Meier method and compared by the log-rank test. All statistical tests were two-sided and differences were considered significant when P < 0.05.
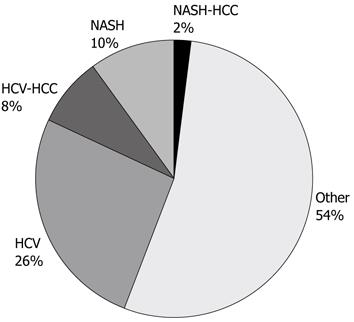
A total of 832 liver transplants were performed at the London Health Sciences Centre during the study period. Of these, 283 (34.0%) recipients were positive for HCV based on the aforementioned criteria. NASH was the indication for liver transplantation in 96 (11.5%) recipients, and 42 (5.1%) recipients were diagnosed with ‘cryptogenic’ or ‘idiopathic’ cirrhosis. The remaining 411 (49.4%) recipients had liver failure due to other identifiable causes. Of the 42 cases originally diagnosed as cryptogenic cirrhosis, 6 were re-designated as NASH associated cirrhosis based on current histologic and clinical definitions. Thus the final analysis of HCV and NASH liver transplant recipients was: 283 (34.0%), 102 (12.3%) respectively (Figure 1).
The incidence of HCC in NASH recipients was 16.7% (17/102). Importantly, none of the re-categorized NASH patients who were originally designated cryptogenic were found to have HCC. The incidence of HCC in HCV liver transplant recipients at our centre was 22.6% (64/283).
Patients with NASH-HCC were statistically older than HCV-HCC patients (58.6 ± 4.2 years vs 52.6 ± 5.8 years, P < 0.001). There was no significant difference in gender or preoperative AFP level between the two groups. No patients with NASH-HCC received a DCD liver allograft (Table 1). The diagnosis of HCC was made before liver transplantation using multiple imaging techniques in 65% of NASH patients and 89% of HCV patients. HCC was more likely to be found incidentally in transplanted NASH patients (35%) than in transplanted HCV patients (11%) (P = 0.015).
| HCV/HCC | NASH/HCC | P value | |
| n = 64 | n = 17 | ||
| Age at transplant (mean ± SD) | 52.6 ± 5.8 | 58.6 ± 4.2 | < 0.001 |
| Gender (% male) | 94% | 94% | 1.000 |
| Donor source (DBD/DCD/LD) | 56/8/0 | 16/0/1 | NA |
| AFP (mean ± SD) | 93.1 ± 204.4 | 20.3 ± 34.0 | 0.149 |
| Number of tumours (mean ± SD) | 1.59 ± 0.81 | 1.64 ± 0.75 | 0.819 |
| Cumulative size of tumours (mean ± SD) | 3.98 ± 2.4 | 3.27 ± 2.1 | 0.270 |
| Vascular invasion | 23.40% | 6.30% | 0.002 |
| Poorly differentiated | 4.70% | 0% | < 0.001 |
Pathological characteristics of the NASH-HCC tumours were compared with those of HCV-HCC tumours (Table 1). A significantly higher proportion of HCV-HCC patients had vascular invasion (23.4% vs 6.4%, P = 0.002) as well as poorly differentiated tumours (4.7% vs 0%, P < 0.001) compared to the NASH-HCC. There was no significant difference in the mean number of tumours or the mean cumulative size of the tumours between the two groups. In both groups, the tumours satisfied Milan criteria pre-transplantation.
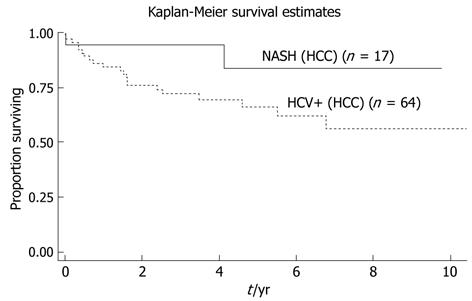
Disease free survival at time of maximal follow-up was not statistically significant between the two groups however there was a clear trend towards lower disease free survival in the HCV-HCC group (P = 0.11, Figure 2).
The prevalence of NAFLD has continued to increase as the obesity epidemic continues. The rate of progression from NAFLD to the development of NASH or end stage liver disease is unknown. However, the frequency of NASH in patients listed for transplantation in North America has been previously determined to be 2.9%[17]. This is likely an underestimate as this number was based on data collected from the 1990s, whereas in the last decade the rates of obesity and metabolic syndrome have increased dramatically. A more recent analysis of data from the Scientific Registry of Transplant Recipients (SRTR) reported that the rate had increased to 3.5%[18]. In our series, 12.3% of patients have NASH as the diagnosis leading to liver failure requiring transplantation. NASH as a primary diagnosis in patients being listed or transplantation has continued to increase at our centre.
The natural history of NAFLD ranging from reversible steatosis to steatohepatitis with hepatic fibrosis (NASH), and ultimately the possibility of developing HCC has been previously described[19]. In small previously published North American series of patients transplanted for NASH, HCC was found in 22% (2/9) of patients[20]. In our series of 102 patients with NASH cirrhosis, 16.7% had HCC at the time of transplantation. This is a similar rate to the 22.6% of our HCV cirrhotic patients requiring transplantation, another well known high risk group for developing HCC. The high incidence of HCC in NASH patients undergoing liver transplantation suggests that these patients are at high risk of developing HCC and should undergo frequent ultrasound surveillance in a similar fashion to that performed in patients with HCV.
It has also been shown that patients with NASH are less likely to be listed for transplantation due to comorbidities, a justifiable practice given suboptimal results in NASH patients possessing theses comorbidities[10]. However in selected NASH patients receiving liver transplantation, others have shown that 5 year disease free survival after transplantation was significantly better than disease free survival in the C and B viral groups, 66%, 29% and 39% respectively[21]. More recent studies have suggested there is no difference in 1 year and 3 year survival between patients transplanted for NASH and those transplanted for other indications[22]. The outcome in patients with HCV is clearly affected by possible recurrence of HCV cirrhosis however NASH patients are also at risk for recurrence of NASH cirrhosis with some studies suggesting the incidence of recurrent NASH being as high as 25%[23-25].
Patients with NASH and HCC post-transplant outcomes have not been previously investigated compared to patients with HCV and HCC (another group in which HCC is commonly seen). In previous studies looking at patients undergoing liver resection for HCC, cumulative survival after resection was comparable among HCV-HCC and NASH-HCC patient groups[21].
In our study a significantly higher proportion of HCV-HCC patients had vascular invasion as well as poorly differentiated tumours compared to the NASH-HCC group. Vascular invasion is the strongest predictor of recurrence in patients with HCC[26].
In the present study a trend of poorer recurrence free survival was seen in HCV-HCC patients compared to NASH-HCC who underwent a Liver transplantation. The higher proportion of patients with vascular invasion and poorly differentiated tumours may at least in part account for this difference in recurrence free survival. Based on our results it therefore appears that NASH associated HCC might be a less aggressive form of HCC compared with HCV associated HCC. It must also be entertained that some of the sickest NASH patients may not be listed for transplantation because of significant comorbidities or poor operative candidacy.
Limitations of the present study include its single centre nature as well as the lack of generalizability to non-transplant NASH and HCC populations due to the unique social and biologic factors of patients approved for transplantation.
In summary in those where NASH progresses to cirrhosis, there is a significant proportion that go on to develop HCC suggesting these individuals should undergo aggressive screening protocols directed toward the early detection of HCC, in a similar fashion to patients with HCV-cirrhosis. Patients with NASH-HCC undergoing liver transplantation also appear to be older than HCV-HCC patients undergoing liver transplantation. In appropriately selected patients with NASH and HCC post-transplant outcomes equal if not better than patients with HCV-HCC (another group in which HCC is commonly seen). This may be related to less vascular invasion and less poorly differentiated pathology.
There is speculation that Non-alcoholic steatohepatitis (NASH) may soon become one of the main causes of end stage liver disease requiring liver transplantation in North America. The clinical outcome and pathologic features of NASH patients with hepatocellular carcinoma (HCC) undergoing liver transplantation compared with hepatitic c virus (HCV) patients with HCC (another group in which HCC is commonly seen) has not been thoroughly investigated.
A significantly higher proportion of HCV-HCC patients had vascular invasion (23.4% vs 6.4%) and poorly differentiated HCC (4.7% vs 0%) compared to the NASH-HCC group. A trend of poorer recurrence free survival at 5 years was seen in HCV-HCC patients compared to NASH-HCC who underwent a Liver transplantation.
To knowledge,this represents the first study to compare the tumour characteristics in explanted livers and disease free survival between NASH-HCC and HCV-HCC patients undergoing liver transplantation.
In appropriately selected patients with NASH and HCC post-transplant outcomes equal if not better than patients with HCV HCC (another group in which HCC is commonly seen). This may be related to less vascular invasion and less poorly differentiated pathology.
In this manuscript, the authors investigated the clinical outcome and pathologic features of NASH patients with HCC undergoing liver transplantation compared with HCV patients with HCC. This is a retrospective analysis. The originality of this study was not so high. Nonetheless, the data were properly presented and the manuscript was well prepared. The results of this study may provide useful information to the clinicians.
Peer reviewer: Wan-Long Chuang, Professor, Internal Medicine, Kaohsiung Medical University, No. 100, Shih-Chuan 1st Road, Kaohsiung 807, Taiwan, China
S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549-1555. [PubMed] [Cited in This Article: ] |
| 2. | Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 886] [Cited by in F6Publishing: 886] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 3. | Ong JP, Younossi ZM. Nonalcoholic fatty liver disease (NAFLD)--two decades later: are we smarter about its natural history? Am J Gastroenterol. 2003;98:1915-1917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42:132-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 647] [Cited by in F6Publishing: 641] [Article Influence: 32.1] [Reference Citation Analysis (1)] |
| 5. | Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol. 2003;98:2042-2047. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 339] [Cited by in F6Publishing: 322] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 6. | Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202-1219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1488] [Cited by in F6Publishing: 1451] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 7. | Charlton M. Nonalcoholic fatty liver disease: a review of current understanding and future impact. Clin Gastroenterol Hepatol. 2004;2:1048-1058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 272] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 8. | Shimada M, Hashimoto E, Taniai M, Hasegawa K, Okuda H, Hayashi N, Takasaki K, Ludwig J. Hepatocellular carcinoma in patients with non-alcoholic steatohepatitis. J Hepatol. 2002;37:154-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 223] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 9. | Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Hepatitis C infection and the increasing incidence of hepatocellular carcinoma: a population-based study. Gastroenterology. 2004;127:1372-1380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 354] [Cited by in F6Publishing: 385] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 10. | O'Leary JG, Landaverde C, Jennings L, Goldstein RM, Davis GL. Patients with NASH and cryptogenic cirrhosis are less likely than those with hepatitis C to receive liver transplants. Clin Gastroenterol Hepatol. 2011;9:700-704.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Malik SM, deVera ME, Fontes P, Shaikh O, Ahmad J. Outcome after liver transplantation for NASH cirrhosis. Am J Transplant. 2009;9:782-793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 12. | Nakano M. Histological study on the resemblance and difference between non-alcoholic steatohepatitis (NASH) and alcoholic liver diseases (ALD). Alcohol Clin Exp Res. 2005;29:230S-235S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Hübscher SG. Role of liver biopsy in the assessment of non-alcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2004;16:1107-1115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5110] [Cited by in F6Publishing: 5195] [Article Influence: 179.1] [Reference Citation Analysis (0)] |
| 15. | Hernandez-Alejandro R, Croome KP, Quan D, Mawardi M, Chandok N, Dale C, McAlister V, Levstik MA, Wall W, Marotta P. Increased risk of severe recurrence of hepatitis C virus in liver transplant recipients of donation after cardiac death allografts. Transplantation. 2011;92:686-689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462-503. [PubMed] [Cited in This Article: ] |
| 17. | Charlton M, Kasparova P, Weston S, Lindor K, Maor-Kendler Y, Wiesner RH, Rosen CB, Batts KP. Frequency of nonalcoholic steatohepatitis as a cause of advanced liver disease. Liver Transpl. 2001;7:608-614. [PubMed] [Cited in This Article: ] |
| 18. | Angulo P. Nonalcoholic fatty liver disease and liver transplantation. Liver Transpl. 2006;12:523-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2065] [Cited by in F6Publishing: 2222] [Article Influence: 158.7] [Reference Citation Analysis (0)] |
| 20. | Ayata G, Gordon FD, Lewis WD, Pomfret E, Pomposelli JJ, Jenkins RL, Khettry U. Cryptogenic cirrhosis: clinicopathologic findings at and after liver transplantation. Hum Pathol. 2002;33:1098-1104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 132] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Wakai T, Shirai Y, Sakata J, Korita PV, Ajioka Y, Hatakeyama K. Surgical outcomes for hepatocellular carcinoma in nonalcoholic fatty liver disease. J Gastrointest Surg. 2011;15:1450-1458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249-1253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 835] [Cited by in F6Publishing: 834] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 23. | El-Masry M, Puig CA, Saab S. Recurrence of non-viral liver disease after orthotopic liver transplantation. Liver Int. 2011;31:291-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Maor-Kendler Y, Batts KP, Burgart LJ, Wiesner RH, Krom RA, Rosen CB, Charlton MR. Comparative allograft histology after liver transplantation for cryptogenic cirrhosis, alcohol, hepatitis C, and cholestatic liver diseases. Transplantation. 2000;70:292-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 62] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Kim WR, Poterucha JJ, Porayko MK, Dickson ER, Steers JL, Wiesner RH. Recurrence of nonalcoholic steatohepatitis following liver transplantation. Transplantation. 1996;62:1802-1805. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 130] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 662] [Cited by in F6Publishing: 649] [Article Influence: 32.5] [Reference Citation Analysis (0)] |