Published online Jan 21, 2012. doi: 10.3748/wjg.v18.i3.237
Revised: September 8, 2011
Accepted: October 28, 2011
Published online: January 21, 2012
AIM: To clarify the relationship between age, menopause, and nonalcoholic fatty liver disease (NAFLD) in women.
METHODS: We conducted a follow-up study on nonalcoholic fatty liver disease by using abdominal ultrasonography, and investigated the relationship of age and menopause with the development of NAFLD in women. We followed 1829 women and 2572 men (response rate, 86%) selected in 2001 to represent the non-institutionalized adult population of Gifu, Japan. Data collected included self-reported medical history, lifestyle factors, and menopausal status. The postmenopausal state was defined as beginning 1 year after the cessation of menses. We diagnosed NAFLD with the aid of abdominal ultrasonography by using diagnostic criteria described previously.
RESULTS: The prevalence of NAFLD in women increases with age, but does not alter with age in men. Furthermore, the prevalence of NAFLD in premenopausal women (6%) was lower than that in men (24%) and in postmenopausal women (15%). The associations of the postmenopausal state and hormone replacement therapy with NAFLD were statistically significant in a univariate logistic regression model. At the follow-up examination, 67 women (5%) were newly diagnosed with NAFLD. The incidence of NAFLD was 3.5% (28/802) in premenopausal women, 7.5% (4/53) in menopausal women, 6.1% (24/392) in postmenopausal women, and 5.3% (11/206) in women receiving hormone replacement therapy. The weight gain in premenopausal women was equal to that in postmenopausal women. Metabolic syndrome and weight gain were independent risk factors for NAFLD in pre- and postmenopausal women, but age was an independent risk factor in premenopausal women only.
CONCLUSION: Aging is a risk factor for NAFLD in premenopausal women, independent of weight gain or influence of metabolic syndrome.
- Citation: Hamaguchi M, Kojima T, Ohbora A, Takeda N, Fukui M, Kato T. Aging is a risk factor of nonalcoholic fatty liver disease in premenopausal women. World J Gastroenterol 2012; 18(3): 237-243
- URL: https://www.wjgnet.com/1007-9327/full/v18/i3/237.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i3.237
Nonalcoholic fatty liver disease (NAFLD) is a common clinical condition with histological features that resemble those of alcohol-induced liver injury, but NAFLD occurs in patients who do not consume alcohol[1,2]. NAFLD encompasses a spectrum of conditions ranging from simple steatosis to steatohepatitis (NASH), advanced fibrosis and cirrhosis[1]. It is emerging as the most common chronic liver disease in Western countries and in other parts of the world[3]. The estimated prevalence of NAFLD in the general population in western countries is 20%-40%[3,4]. Frequent association with disturbances of glucose and lipid metabolism often renders NAFLD a “satellite” element of metabolic syndrome[5,6]. Metabolic syndrome is a cluster of metabolic and hemodynamic abnormalities linked with insulin resistance[7]. Metabolic syndrome is a strong predictor of NAFLD, particularly among people of Japanese descent[8]. Epidemiological studies have shown that metabolic syndrome is not rare and that it is a risk factor for cardiovascular disease (CVD) and stroke[9-11]. Previously, we reported that NAFLD was a predictor of CVD among apparently healthy Japanese men and women[12]. The association between NAFLD and future CVD events was independent of conventional cardiovascular risk factors, because vulnerable plaques tend to develop in patients with NAFLD[13].
Sex and age are conventional cardiovascular risk factors. The prevalence of NAFLD is higher in men than in women, particularly in Asians[14]; however, age is a risk factor for NAFLD in women, but not in men[8]. In this study, we performed a subanalysis within the patient group described in a previous report[8] and investigated the relationship of age and menopause with the development of NAFLD in women.
We accessed a database from a previously reported study[8] to evaluate the possibility that aging and menopause in women could be risk factors for NAFLD. We conducted a follow-up study on nonalcoholic fatty liver disease by using abdominal ultrasonography. All participants who were examined in health checkup programs between January and December 2001 were invited to enroll in the study and 1829 women and 2572 men that satisfied the inclusion criteria in 2001 were selected to represent the non-institutionalized adult population of Gifu, Japan. By the end of June 2003, 3876 of the 4401 participants (2271 men and 1605 women) had completed the second examination (response rate, 86%). The interval between the baseline and follow-up examinations was 414 d (SD = 128). Among the 1605 women, 1603 were available for longitudinal examinations.
We collected data from self-reported medical histories, assessments of lifestyle factors [e.g., age, sex, height, weight, smoking habits, alcohol consumption, menopause status, active or previous hormone replacement therapy (HRT)], and the results of health checkup programs (e.g., urinalysis, blood cell counts, blood chemistry, electrocardiography, chest radiography, barium examination of the upper gastrointestinal tract, and abdominal ultrasonography). The postmenopausal state was defined as beginning 1 year after the cessation of menses. Reports of smoking status included active and past smoking. Habits regarding alcohol consumption were evaluated by asking the participants about the amount and type of alcoholic beverages consumed per week and then estimating the mean ethanol intake per day. We defined an alcohol consumer as someone with a mean ethanol intake of more than 20 g/d. A light drinker was defined as someone with a mean ethanol intake of 0-20 g/d. On the questionnaire, participants reported the type, duration, and frequency of their participation in sports or recreational activities[15]. When participants performed any kind of sport regularly at least once a week, we categorized them as regular exercisers[16].
The ATP III proposed the following 5 abnormalities to define metabolic syndrome: (1) abdominal obesity, abdominal circumference ≥ 102 cm for men and ≥ 88 cm for women; (2) elevated serum triglyceride level, ≥ 1.70 mmol/L (≥ 150 mg/dL); (3) decreased HDL cholesterol level, ≥ 1.04 mmol/L (≥ 40 mg/dL) for men and ≥ 1.30 mmol/L (≥ 50 mg/dL) for women; (4) elevated systolic and diastolic blood pressure, ≥ 130/85 mmHg; and (5) elevated fasting glucose level ≥ 6.11 mmol/L (≥ 110 mg/dL)[7]. Because waist measurements were not available for the entire study sample, we substituted a BMI of ≥ 25 kg/m2 for all participants as an index of obesity. A BMI of ≥ 25 kg/m2 has been proposed as a cutoff for the diagnosis of obesity in Asian people[17]. Individuals with ≥ 3 of the 5 abnormalities were considered to have metabolic syndrome.
We diagnosed NAFLD with the aid of abdominal ultrasonography by using diagnostic criteria described previously[18].
The SPSS statistical package, version 11.0.1 J (SPSS, Inc., Chicago, IL) was used for all statistical analyses, and a P value less than 0.05 was considered statistically significant. Because the incidence rate of NAFLD was unknown, a formal sample size estimate was not made a priori. Cases with and without follow-up visits were compared to determine the appropriateness of an analysis exclusively based on participants with complete data. Two groups of participants were compared using the unpaired t-test and the χ2 test. Logistic regression was used to analyze associations between the development of NAFLD and metabolic syndrome, age, and weight gain. Adjusted odds ratios and 95% CIs were calculated. Data were expressed as means and SDs for continuous variables.
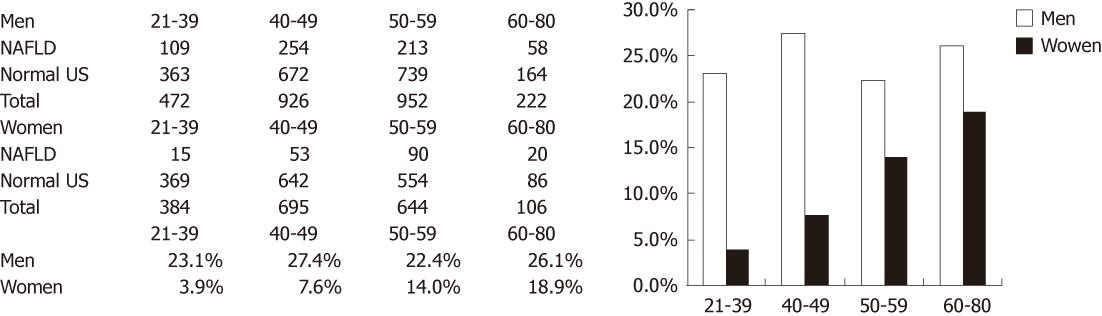
We calculated the age-specific prevalence of NAFLD among 1829 women and 2572 men (Figure 1). The prevalence of NAFLD in women increased with age, but no significant trend for age was identified in men.
We grouped women according to whether they had NAFLD at baseline, and investigated differences in age, weight, components of metabolic syndrome, serum enzymes, menopausal state, alcohol consumption, and smoking history (Table 1). More subjects with NAFLD were postmenopausal, received HRT, and met more criteria of metabolic syndrome than those without NAFLD (Table 1). The prevalence of NAFLD in premenopausal women (6%) was lower than that in men (24%), postmenopausal women (15%), and women receiving HRT (14.0 %, 36/221). In the cross-sectional study, we applied a logistic model to investigate the association of the menopausal state or HRT with NAFLD (Table 2). Univariate analysis showed that the odds ratios of the postmenopausal state and HRT were 2.73 (1.92-3.87), and 2.57 (1.66-3.97), respectively. However, they were not statistically significant after adjusting for age and metabolic syndrome.
| Women w/o NAFLD | Women with NAFLD | P value | Men w/o NAFLD | Men with NAFLD | P value | |
| Number of subjects | 1651 | 178 | 1938 | 634 | ||
| Age, yr (SD) | 46.6 (8.8) | 51.0 (7.7) | < 0.001 | 48.1 (9) | 47.9 (8.4) | 0.66 |
| Body mass index, kg/m2 (SD) | 21.3 (2.5) | 25.7 (3.7) | < 0.001 | 22.5 (2.5) | 25.6 (2.8) | < 0.001 |
| Fasting plasma glucose, mg/mL (SD) | 92.7 (8.4) | 100.7 (10.7) | < 0.001 | 99.6 (13.3) | 106.8 (18.8) | < 0.001 |
| Systolic blood pressure, mmHg (SD) | 111.8 (15.9) | 127.8 (18.2) | < 0.001 | 118.8 (15.8) | 126.4 (15.5) | < 0.001 |
| Diastolic blood pressure, mmHg (SD) | 69.8 (10.1) | 79.1 (10.1) | < 0.001 | 75.3 (10.1) | 80.4 (9.9) | < 0.001 |
| HDL-cholesterol, mg/dL (SD) | 59.4 (13.9) | 49.5 (11.8) | < 0.001 | 47.5 (12.2) | 40.6 (9.7) | < 0.001 |
| Triglycerides, mg/dL (SD) | 73.8 (36.1) | 119.6 (61.4) | < 0.001 | 105.5 (59.9) | 156.7 (89.2) | < 0.001 |
| LDL-cholesterol, mg/dL (SD) | 131.8 (32.2) | 151.1 (33.4) | < 0.001 | 137.2 (30.7) | 145.8 (31.7) | < 0.001 |
| AST, IU/mL (SD) | 15.1 (5.2) | 19.5 (7.9) | < 0.001 | 16.4 (7.7) | 22.8 (10.9) | < 0.001 |
| ALT, IU/mL (SD) | 16.8 (6.5) | 27.9 (15.7) | < 0.001 | 23.1 (15) | 39.6 (21.4) | < 0.001 |
| γGTP, IU/mL (SD) | 11.7 (9.6) | 18.9 (15.4) | < 0.001 | 22.8 (22.8) | 31.4 (22.5) | < 0.001 |
| Hemoglobin, g/dL (SD) | 12.8 (1.2) | 13.3 (1.2) | < 0.001 | 15 (1) | 15.6 (0.9) | < 0.001 |
| C reactive protein, mg/mL (SD) | 0.1 (0.2) | 0.2 (0.4) | 0.113 | 0.1 (0.2) | 0.1 (0.1) | 0.242 |
| Number (%) of light-drinkers | 775 (46.9) | 73 (41.0) | 0.13 | 1513 (78) | 464 (73) | 0.013 |
| Number (%) of current smokers | 102 (6.2) | 10 (5.6) | 0.87 | 795 (41.0) | 234 (36.9) | 0.074 |
| Number (%) of ex-smokers | 184 (11.1) | 12 (6.7) | 0.074 | 555 (28.6) | 213 (33.6) | 0.02 |
| Number (%) of regular exercisers | 340 (20.6) | 27 (15.2) | 0.09 | 393 (20.3) | 78 (12.3) | < 0.001 |
| Number (%) of postmenopausal women | 487 (29.5) | 82 (46.1) | < 0.001 | |||
| Number (%) receiving hormone replacement therapy | 221 (13.4) | 36 (20.2) | 0.017 |
| Unadjusted odds ratio (95% CI) | P values | Adjusted odds ratio (95% CI) | P values | |
| Age | 1.06 (1.04-1.08) | < 0.001 | 1.03 (1-1.06) | 0.027 |
| Postmenopausal state | 2.73 (1.92-3.87) | < 0.001 | 1.55 (0.92-2.61) | 0.096 |
| Active hormone replacement therapy | 2.57 (1.66-3.97) | < 0.001 | 1.54 (0.93-2.54) | 0.092 |
| Presence of metabolic syndrome at baseline | 12.52 (8.03-19.51) | < 0.001 | 11.28 (7.04-18.06) | < 0.001 |
Next, we excluded 257 subjects with a history of hysterectomy or HRT. We separated the subjects into pre- or postmenopausal groups and investigated the difference in age, components of metabolic syndrome, alcohol consumption, and smoking history (Table 3). An association of age with NAFLD was identified in premenopausal women, but not in postmenopausal women. On the other hand, an association of the components of metabolic syndrome with NAFLD was identified in both groups.
| Premenopausal women (n = 1023) | Postmenopausal women (n = 549) | |||||
| Normal US | NAFLD | P Value | Normal US | NAFLD | P Value | |
| Number of subjects (%) | 962 | 61 (6) | 468 | 81 (15) | ||
| Mean age ± SD, yr | 42.2 (6.4) | 44.7 (6.1) | 0.003 | 56 (5.5) | 55.9 (5.4) | 0.874 |
| Number (%) who met a criterion of metabolic syndrome | ||||||
| Body mass index | 75 (8) | 32 (52) | < 0.001 | 37 (8) | 43 (53) | < 0.001 |
| Glucose | 24 (2) | 15 (25) | < 0.001 | 25 (5) | 8 (10) | < 0.001 |
| Blood pressure | 94 (10) | 21 (34) | < 0.001 | 104 (22) | 35 (43) | < 0.001 |
| HDL-cholesterol | 221 (23) | 31 (51) | < 0.001 | 128 (27) | 48 (59) | < 0.001 |
| Triglycerides | 29 (3) | 13 (21) | < 0.001 | 36 (8) | 21 (26) | < 0.001 |
| Number (%) who met ≥3 criteria of metabolic syndrome | 18 (2) | 17 (28) | < 0.001 | 26 (6) | 23 (28) | < 0.001 |
| Number (%) light-drinkers | 472 (49.1) | 24 (3.9) | 0.15 | 190 (40.6) | 34 (42.0) | 0.81 |
| Number (%) current smokers | 48 (5.0) | 4 (6.6) | 0.54 | 34 (7.3) | 5 (6.2) | 0.64 |
| Number (%) ex-smokers | 103 (1.1) | 5 (8.2) | 0.67 | 50 (10.7) | 5 (6.2) | 0.31 |
| Number (%) regular exercisers | 181 (18.8) | 8 (13.1) | 0.31 | 105 (22.4) | 15 (18.5) | 0.47 |
Of the 1603 women who completed follow up, 1453 women did not have NAFLD at baseline. Of the 1453 women who were disease-free at the baseline examination and who had the follow-up examination, 67 women (5%) received new diagnoses of NAFLD at follow-up examination. Subjects with NAFLD were older, showed more weight gain, and met more criteria for metabolic syndrome than those without NAFLD, but the 2 groups showed no differences in menopausal state, HRT, alcohol consumption, and smoking history.
Among the 1453 disease-free women at baseline, there were 855 premenopausal women and 392 postmenopausal women. Of the 855 premenopausal women, 53 women were menopausal. During the study period, 206 women received HRT. The incidence of NAFLD was 3.5% (28/802) in premenopausal women, 7.5% (4/53) in menopausal women, 6.1% (24/392) in postmenopausal women, and 5.3% (11/206) in women receiving HRT.
We applied a logistic regression model to determine the risk factors for the development of NAFLD (Table 4). Univariate analysis indicated that the postmenopausal state was a risk factor for NAFLD [odds ratio 1.8 (1.03-3.15), P = 0.039], but it was not statistically significant after adjusting for age, metabolic syndrome, and weight gain. The cross-sectional study at baseline revealed that the prevalence of NAFLD was higher in women receiving HRT than in those in the premenopausal state, but the logistic model did not indicate that HRT was a risk factor for the development of NAFLD.
| Unadjusted odds ratio (95% CI) | P values | Adjusted odds ratio (95% CI) | P values | |
| Age | 1.05 (1.02-1.08) | 0.001 | 1.06 (1.02-1.1) | 0.004 |
| Menopause | 2.26 (0.76-6.69) | 0.14 | 1.22 (0.38-3.99) | 0.74 |
| Postmenopausal state | 1.8 (1.03-3.15) | 0.039 | 0.72 (0.31-1.66) | 0.44 |
| Active hormone replacement therapy | 1.56 (0.76-3.19) | 0.22 | 1.32 (0.6-2.88) | 0.49 |
| Presence of metabolic syndrome at baseline | 9.89 (4.67-20.94) | < 0.001 | 11.7 (5.02-27.25) | < 0.001 |
| Weight gain | 1.50 (1.30-1.74) | < 0.001 | 1.63 (1.39-1.9) | < 0.001 |
Next, we excluded 206 women with HRT at the baseline and/or follow up. We separated the subjects into pre- or postmenopausal groups and compared parameters in subjects with NAFLD to those without (Table 5). Weight gain was equal in pre- and postmenopausal women. There was an association of the number of women who met ≥3 criteria of metabolic syndrome and weight gain with the development of NAFLD in pre- and postmenopausal women, but an association of age with development of NAFLD was identified in premenopausal subjects only.
| Premenopausal women (n = 855) | Postmenopausal women (n = 392) | |||||
| Normal US at both baseline and follow-up | Normal US at baseline and NAFLD at follow-up | P value | Normal US at both baseline and follow-up | Normal US at baseline and NAFLD at follow-up | P value | |
| Number (% incidence of NAFLD) | 823 | 32 (4) | 368 | 24 (6) | ||
| Mean age ± SD, yr (% incidence of NAFLD) | 42.2 (6.3) | 45.5 (6) | 0.004 | 55.7 (5.6) | 55.7 (4.7) | 0.951 |
| Number (%) who met a criterion of metabolic syndrome | ||||||
| Body mass index | 53 (6) | 13 (41) | < 0.001 | 28 (8) | 4 (17) | 0.12 |
| Glucose | 18 (2) | 5 (16) | 0.001 | 19 (5) | 3 (13) | 0.14 |
| Blood pressure | 79 (10) | 9 (28) | 0.003 | 79 (21) | 8 (33) | 0.2 |
| HDL-cholesterol | 178 (22) | 19 (59) | < 0.001 | 98 (27) | 10 (42) | 0.15 |
| Triglycerides | 18 (2) | 7 (22) | < 0.001 | 25 (7) | 4 (17) | 0.091 |
| Number (%) who met ≥ 3 criteria of metabolic syndrome | 9 (1) | 7 (22) | < 0.001 | 17 (5) | 4 (17) | 0.032 |
| Weight gain, kg (SD) | 0.2 (1.7) | 1.4 (1.6) | < 0.001 | 0 (1.6) | 1.3 (1.3) | < 0.001 |
| Number (%) light-drinkers | 406 (49.3) | 14 (43.8) | 0.59 | 214 (26.0) | 10 (31.3) | 1 |
| Number (%) current smokers | 42 (5.1) | 3 (9.4) | 0.23 | 38 (4.6) | 0 (0.0) | 0.4 |
| Number (%) ex-smokers | 88 (10.7) | 6 (18.8) | 0.15 | 54 (6.6) | 1 (3.1) | 0.5 |
| Number (%) regular exercisers | 151 (18.3) | 6 (18.8) | 1 | 81 (22.0) | 4 (16.7) | 0.78 |
Finally, we applied a multivariate logistic model to investigate the risk of NAFLD after separating women into pre- or postmenopausal groups (Table 6). Metabolic syndrome and weight gain were dependent risk factors for NAFLD in pre- and postmenopausal women, but age was a risk factor only in premenopausal women.
| Premenopausal subjects (n = 855) | Postmenopausal subjects (n = 392) | |||
| Adjusted odds ratio (95% CI) | P Value | Adjusted odds ratio (95% CI) | P Value | |
| Age | 1.12 (1.05–1.2) | 0.001 | 1 (0.93–1.07) | 0.91 |
| Presence of metabolic syndrome at baseline | 43.06 (12.66–146.51) | < 0.001 | 4.87 (1.29–18.34) | 0.019 |
| Weight gain | 1.76 (1.4–2.21) | < 0.001 | 1.67 (1.27–2.21) | < 0.001 |
Both NAFLD and NASH are reported to be more prevalent in men than in women[19,20]. Our study indicates that the prevalence of NAFLD in women increases with age, but does not alter with age in men. Furthermore, the prevalence of NAFLD in premenopausal women (6%) was lower than that in men (24%) and in postmenopausal women (15%). A univariate logistic regression model indicated that when no adjustments were made for age or metabolic syndrome, the associations of the postmenopausal state and HRT with NAFLD were statistically significant.
The present prospective study revealed that the incidence of NAFLD was highest in women in menopause (7.5%, 4/53). The incidence of NAFLD was higher in postmenopausal women (6.1%, 24/392) or in women with HRT (5.3%, 11/206) than in premenopausal women (3.5%, 28/802). Our findings are in agreement with recent cross-sectional studies, which indicate that NAFLD is more prevalent in postmenopausal women than in premenopausal women[21-23].
The univariate logistic regression model indicated that the postmenopausal state was a risk factor for NAFLD, but this was not statistically significant after adjusting for age, metabolic syndrome, and weight gain. Age, metabolic syndrome, and body weight gain were independent risk factors for NAFLD in women. However, after separating women into pre- or postmenopausal groups, age remained the only independent risk factor in premenopausal women. These results imply that aging increases the risk for NAFLD in premenopausal women, but poses no risk after menopause; thus, the influence of aging in postmenopausal women is similar to that in men. Furthermore, the proportion of premenopausal women with NAFLD was smaller than that in men, but the proportion of postmenopausal women with NAFLD was equal to that in men.
A possible underlying factor is estrogen-related sex hormones. Estrogens lead to preferential fat accumulation in the gluteofemoral region, and the loss of estrogens during the transition of menopause is associated with an increase in central fat[22]. Gutierrez-Grobe et al[21] reported that women without NAFLD had significantly higher levels of serum estradiol, which is a major form of estrogen, than those with NAFLD both at pre- and postmenopausal stages. Hepatic estrogen receptors mediate estrogen action in the liver, and estradiol has a favorable role in chronic liver disease[24], which is suggestive of a protective effect of estrogens against NAFLD in women[21]. The relationship between aging in premenopausal women and the development of NAFLD may thus reflect changes in levels of estrogen-related sex hormones. Unfortunately, we did not measure the levels of estrogen-related sex hormones in this study.
In the present cross-sectional study, we found that NAFLD was more prevalent in women receiving HRT than in premenopausal women, as reported elsewhere[23]. However, the logistic model did not indicate that HRT was a risk factor for NAFLD. The protective effect of estrogen-related sex hormones might be limited to endogenous estrogens. However, since the present prospective study was observational, several background factors, including age, differed between the HRT and non-HRT groups. A recent study suggested that HRT improved the results of liver-function tests[25]. Moreover, tamoxifen, an anti-estrogen compound used in the treatment of estrogen receptor positive breast cancer, is associated with an increased risk of fatty liver and NASH[26,27]. An animal study showed that hepatic steatosis occurs spontaneously in aromatase-deficient mice who lack the ability to produce estrogen and who have impaired hepatocellular lipid β-oxidation[28]. Estradiol replacement reduces hepatic steatosis and restores the impairment in mitochondrial and peroxisomal fatty acid β-oxidation to the level found in wild-type mice[29]. Thus, a well-designed randomized controlled study is required to determine the influence of HRT on NAFLD.
Recent studies have shown that visceral fat is an independent predictor of fatty liver, even in patients with normal BMI, and is much more harmful than subcutaneous accumulation of adipose tissue[30,31]. We demonstrated that metabolic syndrome and weight gain are risk factors of NAFLD[8] and evaluated these factors in pre- and postmenopausal women. The number of women who met the criteria for metabolic syndrome was larger among postmenopausal women, but weight gain was the same in both.
We note the following limitations in our study. We assessed active and past medical history, state of menses, smoking habits, drinking habits, and physical activities by conducting interviews. A trained nurse supported the interviewer, but we cannot exclude the possibility of interviewer bias. Furthermore, information on dietary composition was lacking.
In conclusion, aging is a risk factor for NAFLD in premenopausal women, but is not a risk factor in postmenopausal women or in men. This is one of the reasons that the number of women with NAFLD increases with age, and reaches the maximum level in women in their sixties.
We thank all staff members in the medical health checkup center, Murakami Memorial Hospital.
Nonalcoholic fatty liver disease (NAFLD) is emerging as the most common chronic liver disease. NAFLD is more common in men than women, particularly in Asians.
The prevalence of NAFLD in women increases with age, but not in men. Age and menopause may be risk factors for the incidence of NAFLD. But, this has not been clarified yet.
The prevalence of NAFLD was lower in premenopausal women than in men, but in postmenopausal women, the prevalence of NAFLD was similar to that in men. The prospective study indicated that aging is a risk factor for the development of NAFLD in premenopausal women independent of weight gain or metabolic syndrome.
The study suggests the possibility that premenopausal women have a protective factor against NAFLD, which is weakened by aging.
NAFLD was less prevalent in females than in males, but only before menopause. The prevalence of NAFLD was lower in women than in men, and increased with age in women, but a significant trend for age was not identified in men. NAFLD was independently associated with BMI and metabolic syndrome in both pre- and postmenopausal women, but with age only in premenopausal women. The results are interesting and suggest that the premenopausal state may be associated with a protective factor against NAFLD, which weakens with age.
Peer reviewers: Kazuaki Takabe, MD, PhD, Assistant Professor of Surgery and Assistant Professor of Biochemistry and Molecular Biology, Surgical Oncology, VCU Massey Cancer Center, Virginia Commonwealth University/Medical College of Virginia, PO Box 980011, Richmond VA 23298-0011, United States; Dr. Luca Valenti, Internal Medicine, Università degli Studi di Milano, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, via Francesco Sforza 35, padiglione Granelli, Milano 20122, Italy; Dr. Nagarajan Perumal, Compliance veterinarian, Center for life science, IACUC OFFICE, National University of Singapore, 117456, Singapore
S- Editor Lv S L- Editor O’Neill M E- Editor Xiong L
| 1. | Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467-2474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Mulhall BP, Ong JP, Younossi ZM. Non-alcoholic fatty liver disease: an overview. J Gastroenterol Hepatol. 2002;17:1136-1143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 141] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 3. | Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649-1657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 644] [Cited by in F6Publishing: 633] [Article Influence: 28.8] [Reference Citation Analysis (2)] |
| 4. | Grattagliano I, Portincasa P, Palmieri VO, Palasciano G. Managing nonalcoholic fatty liver disease: recommendations for family physicians. Can Fam Physician. 2007;53:857-863. [PubMed] [Cited in This Article: ] |
| 5. | Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, Forlani G, Melchionda N. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450-455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1074] [Cited by in F6Publishing: 1067] [Article Influence: 42.7] [Reference Citation Analysis (1)] |
| 6. | Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 882] [Cited by in F6Publishing: 878] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 7. | Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497. [PubMed] [Cited in This Article: ] |
| 8. | Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, Omatsu T, Nakajima T, Sarui H, Shimazaki M. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:722-728. [PubMed] [Cited in This Article: ] |
| 9. | Meigs JB. Epidemiology of the metabolic syndrome, 2002. Am J Manag Care. 2002;8:S283-S92; quiz S283-S92;. [PubMed] [Cited in This Article: ] |
| 10. | Sattar N, Gaw A, Scherbakova O, Ford I, O'Reilly DS, Haffner SM, Isles C, Macfarlane PW, Packard CJ, Cobbe SM. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation. 2003;108:414-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1072] [Cited by in F6Publishing: 1014] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 11. | Ninomiya JK, L'Italien G, Criqui MH, Whyte JL, Gamst A, Chen RS. Association of the metabolic syndrome with history of myocardial infarction and stroke in the Third National Health and Nutrition Examination Survey. Circulation. 2004;109:42-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 523] [Cited by in F6Publishing: 567] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 12. | Hamaguchi M, Kojima T, Takeda N, Nagata C, Takeda J, Sarui H, Kawahito Y, Yoshida N, Suetsugu A, Kato T. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol. 2007;13:1579-1584. [PubMed] [Cited in This Article: ] |
| 13. | Akabame S, Hamaguchi M, Tomiyasu K, Tanaka M, Kobayashi-Takenaka Y, Nakano K, Oda Y, Yoshikawa T. Evaluation of vulnerable coronary plaques and non-alcoholic fatty liver disease (NAFLD) by 64-detector multislice computed tomography (MSCT). Circ J. 2008;72:618-625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Weston SR, Leyden W, Murphy R, Bass NM, Bell BP, Manos MM, Terrault NA. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005;41:372-379. [PubMed] [Cited in This Article: ] |
| 15. | Aaron DJ, Kriska AM, Dearwater SR, Cauley JA, Metz KF, LaPorte RE. Reproducibility and validity of an epidemiologic questionnaire to assess past year physical activity in adolescents. Am J Epidemiol. 1995;142:191-201. [PubMed] [Cited in This Article: ] |
| 16. | Ryu S, Chang Y, Kim DI, Kim WS, Suh BS. gamma-Glutamyltransferase as a predictor of chronic kidney disease in nonhypertensive and nondiabetic Korean men. Clin Chem. 2007;53:71-77. [PubMed] [Cited in This Article: ] |
| 17. |
The Asia-Pacific Perspective: Redefining Obesity and Its Treatment. |
| 18. | Hamaguchi M, Kojima T, Itoh Y, Harano Y, Fujii K, Nakajima T, Kato T, Takeda N, Okuda J, Ida K. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol. 2007;102:2708-2715. [PubMed] [Cited in This Article: ] |
| 19. | Weltman MD, Farrell GC, Hall P, Ingelman-Sundberg M, Liddle C. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology. 1998;27:128-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 436] [Cited by in F6Publishing: 423] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 20. | American Gastroenterological Association. American Gastroenterological Association medical position statement: nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1702-1704. [PubMed] [Cited in This Article: ] |
| 21. | Gutierrez-Grobe Y, Ponciano-Rodríguez G, Ramos MH, Uribe M, Méndez-Sánchez N. Prevalence of non alcoholic fatty liver disease in premenopausal, posmenopausal and polycystic ovary syndrome women. The role of estrogens. Ann Hepatol. 2010;9:402-409. [PubMed] [Cited in This Article: ] |
| 22. | Völzke H, Schwarz S, Baumeister SE, Wallaschofski H, Schwahn C, Grabe HJ, Kohlmann T, John U, Dören M. Menopausal status and hepatic steatosis in a general female population. Gut. 2007;56:594-595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Park SH, Jeon WK, Kim SH, Kim HJ, Park DI, Cho YK, Sung IK, Sohn CI, Keum DK, Kim BI. Prevalence and risk factors of non-alcoholic fatty liver disease among Korean adults. J Gastroenterol Hepatol. 2006;21:138-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 299] [Cited by in F6Publishing: 297] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 24. | Shimizu I, Kohno N, Tamaki K, Shono M, Huang HW, He JH, Yao DF. Female hepatology: favorable role of estrogen in chronic liver disease with hepatitis B virus infection. World J Gastroenterol. 2007;13:4295-4305. [PubMed] [Cited in This Article: ] |
| 25. | McKenzie J, Fisher BM, Jaap AJ, Stanley A, Paterson K, Sattar N. Effects of HRT on liver enzyme levels in women with type 2 diabetes: a randomized placebo-controlled trial. Clin Endocrinol (Oxf). 2006;65:40-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Van Hoof M, Rahier J, Horsmans Y. Tamoxifen-induced steatohepatitis. Ann Intern Med. 1996;124:855-856. [PubMed] [Cited in This Article: ] |
| 27. | Oien KA, Moffat D, Curry GW, Dickson J, Habeshaw T, Mills PR, MacSween RN. Cirrhosis with steatohepatitis after adjuvant tamoxifen. Lancet. 1999;353:36-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 86] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Jones ME, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, Oz OK, Leury BJ, Robertson KM, Yao S. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci USA. 2000;97:12735-12740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Nemoto Y, Toda K, Ono M, Fujikawa-Adachi K, Saibara T, Onishi S, Enzan H, Okada T, Shizuta Y. Altered expression of fatty acid-metabolizing enzymes in aromatase-deficient mice. J Clin Invest. 2000;105:1819-1825. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 162] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 30. | Bellentani S, Saccoccio G, Masutti F, Crocè LS, Brandi G, Sasso F, Cristanini G, Tiribelli C. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112-117. [PubMed] [Cited in This Article: ] |
| 31. | Omagari K, Kadokawa Y, Masuda J, Egawa I, Sawa T, Hazama H, Ohba K, Isomoto H, Mizuta Y, Hayashida K. Fatty liver in non-alcoholic non-overweight Japanese adults: incidence and clinical characteristics. J Gastroenterol Hepatol. 2002;17:1098-1105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 204] [Article Influence: 9.3] [Reference Citation Analysis (0)] |