Copyright
©2012 Baishideng Publishing Group Co.
World J Gastroenterol. Jul 28, 2012; 18(28): 3752-3760
Published online Jul 28, 2012. doi: 10.3748/wjg.v18.i28.3752
Published online Jul 28, 2012. doi: 10.3748/wjg.v18.i28.3752
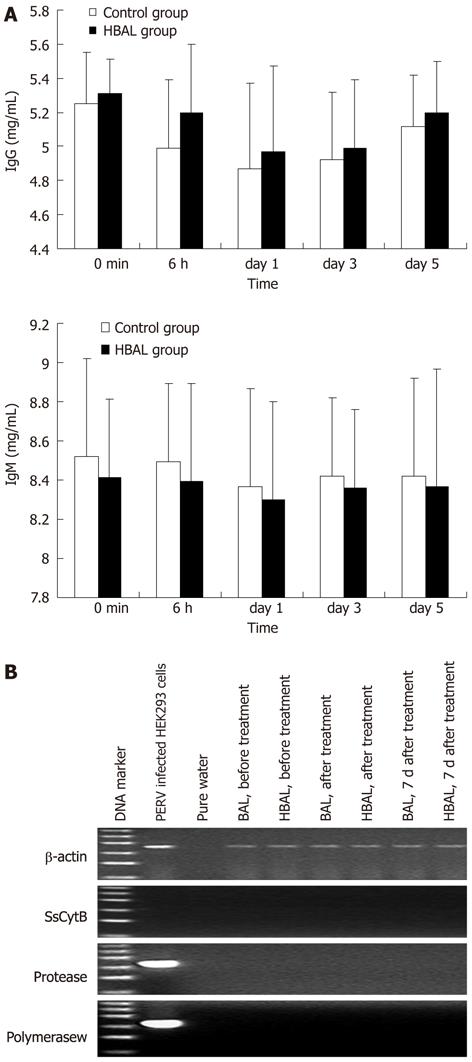
Figure 4 Safety evaluation.
A: Xenoreactive antibodies levels during bioartificial liver treatments; B: Representative results of reverse transcription-polymerase chain reaction electrophoresis with the RNA extracted from the plasma. The ladder ranged from 100 bp to 600 bp. NBAL: Hybrid bioartificial liver; BAL: Bioartificial liver; PERV: Porcine endogenous retrovirus.
- Citation: Shi XL, Zhang Y, Chu XH, Han B, Gu JY, Xiao JQ, Tan JJ, Gu ZZ, Ren HZ, Yuan XW, Ding YT. Evaluation of a novel hybrid bioartificial liver based on a multi-layer flat-plate bioreactor. World J Gastroenterol 2012; 18(28): 3752-3760
- URL: https://www.wjgnet.com/1007-9327/full/v18/i28/3752.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i28.3752