Copyright
©2012 Baishideng Publishing Group Co.
World J Gastroenterol. Jul 28, 2012; 18(28): 3752-3760
Published online Jul 28, 2012. doi: 10.3748/wjg.v18.i28.3752
Published online Jul 28, 2012. doi: 10.3748/wjg.v18.i28.3752
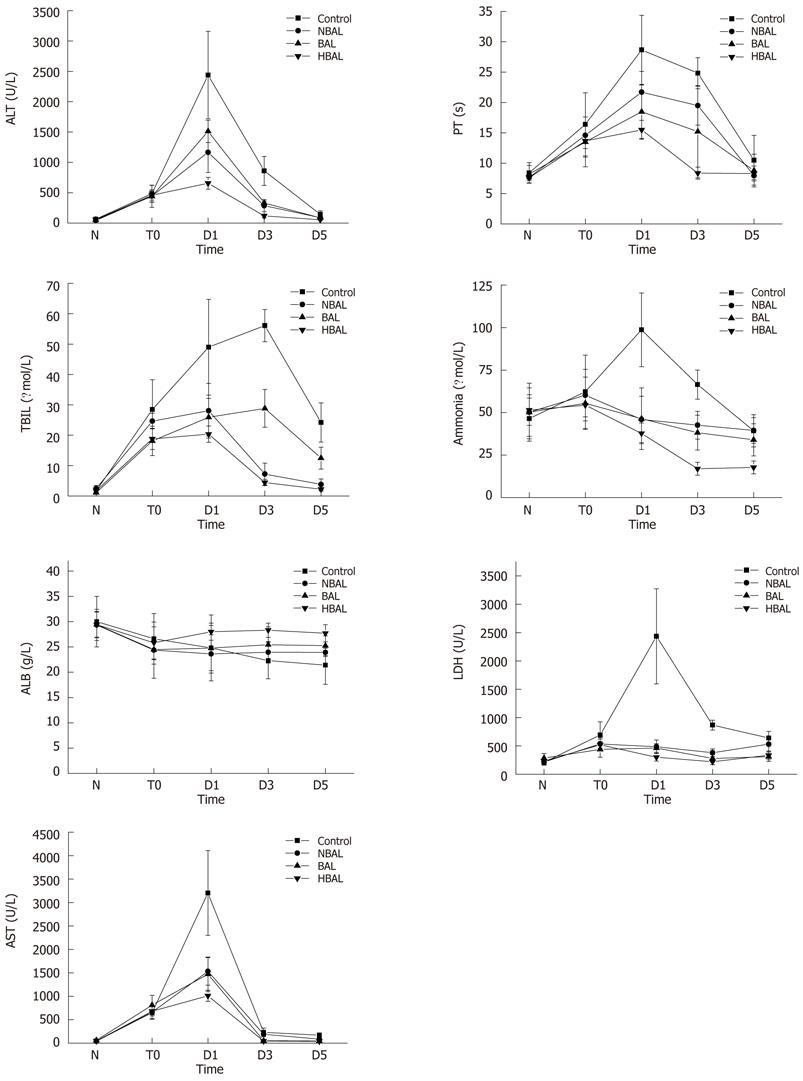
Figure 2 Comparisons of biochemical index between the treatment groups and the control group.
ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; LDH: Lactate dehydrogenase; TBIL: Total bilirubin; ALB: Albumin; PT: Prothrombin time; HBAL: Hybrid bioartificial liver; BAL: Bioartificial liver; NBAL: Non-bioartificial liver; N: Normal; T0: Before treatment; D1: Day 1 after treatment; D3: Day 3 after treatment; D5: Day 5 after treatment.
- Citation: Shi XL, Zhang Y, Chu XH, Han B, Gu JY, Xiao JQ, Tan JJ, Gu ZZ, Ren HZ, Yuan XW, Ding YT. Evaluation of a novel hybrid bioartificial liver based on a multi-layer flat-plate bioreactor. World J Gastroenterol 2012; 18(28): 3752-3760
- URL: https://www.wjgnet.com/1007-9327/full/v18/i28/3752.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i28.3752