Published online Jun 21, 2012. doi: 10.3748/wjg.v18.i23.2995
Revised: March 22, 2012
Accepted: May 6, 2012
Published online: June 21, 2012
AIM: To investigate the intratumoral expression of metastasis-associated in colon cancer 1 (MACC1) and c-Met and determine their clinical values associated with hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC).
METHODS: A retrospective study admitted three hundred fifty-four patients with HBV-related HCC. The expression and distribution of MACC1 and c-Met were assessed by quantitative real-time polymerase chain reaction and immunohistochemistry staining. Prognostic factors influencing survival, metastasis and recurrence were assessed.
RESULTS: Intratumoral MACC1 level was found to be associated with HCC disease progression. Both median tumor-free survival (TFS) and overall survival (OS) were significantly shorter in the postoperative HCC patients with high intratumoral MACC1 expression, as compared to those with low intratumoral MACC1 levels (TFS: 34 mo vs 48.0 mo, P < 0.001; OS: 40 mo vs 48 mo, P < 0.01). Multivariable analysis indicated that high MACC1 expression or co-expression with c-Met were independent predictors for HCC clinic outcome (P < 0.001).
CONCLUSION: High intratumoral MACC1 expression can be associated with enhanced tumor progression and poor outcome of HBV-related HCC. MACC1 may serve as a prognostic biomarker for postoperative HCC.
- Citation: Qu JH, Chang XJ, Lu YY, Bai WL, Chen Y, Zhou L, Zeng Z, Wang CP, An LJ, Hao LY, Xu GL, Gao XD, Lou M, Lv JY, Yang YP. Overexpression of metastasis-associated in colon cancer 1 predicts a poor outcome of hepatitis B virus-related hepatocellular carcinoma. World J Gastroenterol 2012; 18(23): 2995-3003
- URL: https://www.wjgnet.com/1007-9327/full/v18/i23/2995.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i23.2995
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third cause of death from cancers worldwide[1]. The incidence of HCC in China is high, and most cases are associated with chronic hepatitis B virus (HBV) infection[2]. Hepatocarcinogenesis is a complex process associated with the accumulation of multiple genetic and epigenetic changes during the initiation, progression and maturation of this fatal disease[3,4]. As such, intensive research efforts have been carried out to determine the physiological, cellular and molecular mechanisms of HCC, in the hope of developing effective preventative measures and improved treatment strategies.
The metastasis-associated in colon cancer 1 (MACC1) gene was identified by a genome-wide screen of human colon cancer samples, and its expression was closely related to the metastasis of colon cancers[5]. Subsequent clinical studies have suggested that MACC1 might be an important predictor for metastasis and recurrence of colon cancers. Further studies have revealed that MACC1-induced tumorigenesis is correlated with enhanced hepatocyte growth factor (HGF)/c-Met signaling[6,7]. MACC1 functions as a transcription factor, and one of its target promoters is that of the receptor tyrosine kinase c-Met gene. Binding of MACC1 to the promoter has been demonstrated to stimulate c-Met transcription, ultimately inducing activation of the HGF/c-Met signaling pathway and enhancing cell proliferation, motility, and metastasis[8,9]. MACC1 is normally expressed in healthy liver tissues, but marked overexpression is frequently observed in HCC clinical samples[10]. To date, however, the clinical significance of MACC1 overexpression in HCC and of the correlation between MACC1 and the c-Met signaling in the disease state remain unknown. It is intriguing to speculate that MACC1 may contribute to HCC onset and progression, and therefore may represent a readily-detectable biomarker for tumor recurrence and/or metastasis in postoperative HCC patients.
In this study, we sought to determine the expression levels of MACC1 in HBV-related HCC at different disease stages and analyze its correlation with clinical outcome. In addition, we evaluated the related levels of its transcriptional target, c-Met. Our data indicated that MACC1 expression levels represent an effective prognostic factor for HBV-related HCC patients who undergo hepatectomy.
Tumor samples were obtained from 412 consecutive patients admitted to The 302nd Hospital (Beijing, China) with HBV-related HCC from December 2004 to June 2006. The diagnosis of HCC was based on the criteria of the European Association for the Study of the Liver[11]. By using the Barcelona Clinic Liver Cancer (BCLC) staging classification system[12], 148 patients were classified as stage A, 144 as stage B, and 120 as stage C. All patients, except for those at stage C, had undergone surgical resection. Among all patients, 36 had an incomplete resection, 12 died from other causes without recurrence, and 10 were lost to follow-up for non-medical reasons. Thus, the total study population was composed of 354 patients (Table 1). Matched non-tumor tissue samples were obtained from all surgical resected participants and were generally taken from a distance of more than 2 cm from the tumor tissue. Tumor samples from the stage C individuals were obtained by using the Single Action Biopsy Device (Promex Technologies, United States) and target tissues were identified by the following criteria: solitary lesions, or up to three nodules ≤ 6 cm in size; partial portal vein thrombosis or vena cana invasion; absence of extrahepatic metastasis; and preserved liver function (Child-Pugh A or ≤ B8 with serum bilirubin levels under 51.3 μmol/L). In addition, ten normal liver tissues were obtained from four cases of hepatic hemangioma and six patients with hepatic cyst, none of which had a history of viral hepatitis or liver cirrhosis.
| Clinical features | Stage A | Stage B | Stage C |
| Cases (n) | 138 | 96 | 120 |
| Median age (yr, range) | 55 (24-68) | 53 (29-70) | 52 (21-72) |
| Male/female | 118/20 | 82/14 | 106/14 |
| Median tumor diameter (cm, range) | 2.5 (1.5-3) | 4.0 (3-5) | 4.5 (2-6) |
| AFP (μg/L, > 400/ ≤ 400) | 45/93 | 40/56 | 85/35 |
| HBV DNA (+/-) | 78/60 | 57/39 | 76/44 |
| HBeAg (+/-) | 50/88 | 32/64 | 54/66 |
| ECOG PS (0/1/2) | 92/30/16 | 60/25/11 | 25/55/40 |
| Child-Pugh (A/B) | 92/46 | 67/29 | 58/62 |
| Tumor number (single/multinodular) | 123/15 | 56/40 | 67/53 |
| Invasion of portal vein (+/-) | 0/138 | 0/96 | 120/0 |
| Tumor differentiation (high/intermediate/low) | 45/66/27 | 27/38/31 | 20/50/50 |
Each of the samples were divided and either prepared for histochemical staining or snap-frozen in liquid nitrogen for RNA extraction for use in subsequent reverse transcription (RT)-polymerase chain reaction (PCR). For hematoxylin and eosin (HE) and immunohistochemical staining, the tissues were fixed in 10% formalin and paraffin-embedded.
The study protocol was approved by The 302nd Hospital Research Ethics Committee, and written informed consent was obtained from all participants or their legal guardian. None of the patients had received prior treatment for HCC, including radiation or chemotherapy. Patients were followed up every 2 mo within the first postoperative year and at approximately 3-4 mo intervals thereafter. Routine evaluation included physical examination, chest roentgenography, blood chemistry analysis, HBV-DNA test, and measurement of tumor markers (carcinoembryonic antigen and α-fetoprotein). Chest and abdominal computed tomography, brain magnetic resonance imaging and a bone scintiscan were performed every 6 mo for three years after surgery. Additional examinations were performed if any symptoms or signs of recurrence were detected.
The levels of the mRNA transcripts of MACC1 and c-Met were determined by quantitative real-time PCR, as described previously[13]. β-actin mRNA expression was used as an internal control and the relative gene expression values were calculated by the 2-ΔCt method using Sequence Detection System 2.1 software. Total RNA was isolated from the tissues by using an RNA isolation kit (Qiagene, Germany) and following the manufacturer’s instructions. The concentration of RNA was determined by spectrophotometric measurement at A260, and the purity was verified by the A260/A280 ratio (> 1.8 was sufficiently pure). A total of 2 μg RNA was used for the preparation of cDNA by reverse transcriptase-PCR (SYBR PrimeScript RT-PCR Kit with SYBR Premix Ex Taq; Takara, Japan). The following PCR primers were used: MACC1 cDNA (136 bp), 5’-TTCTTTTGATTCCTCCGGTGA-3’ (F) and 5’-ACTCTGATGGGCATGTGCTG-3’ (R); c-Met cDNA (173 bp), 5’-GCAGGTTGTGGTTTCTCG-3’ (F) and 5’-TGCAGCCCAAGCCATTCA-3’ (R); and β-actin cDNA (125 bp), 5’-CGGGAAATCGTGCGTGAC-3’ (F) and 5’-AGGCAGCTCGTAGCTCTTCT-3’ (R). The cDNA equivalent of 50 ng of the original RNA was used in the PCR. The 50 μL reactions for MACC1 or c-Met were run for 40 cycles as follows: predenaturation at 95 °C for 30 s, denaturation at 95 °C for 5 s, annealing and extension at 60 °C for 30 s. The target mRNA was normalized to the corresponding β-actin signal. Measurements were performed in triplicate.
Paraffin-embedded tissues from resected tumor and non-tumor tissues or biopsied tumor samples were cut for serial microtome sections with 4 μm thickness. After hematoxylin and eosin staining, the samples were assessed by two independent pathologists using Edmondson criteria[14]. Samples were classified as: well differentiated, corresponding to Edmondson’s Grade I or I-II; moderately differentiated, corresponding to Edmondson’s Grade II or II-III; or poorly differentiated, corresponding to Edmondson’s Grade III or III-IV.
Two additional serial sections from each individual were prepared for MACC1 and c-Met immunohistochemical staining. Monoclonal rabbit anti-human antibody against MACC1 (1:50; Sigma, United States) and rabbit anti-human antibody against c-Met (1:250; Abcam, Hong Kong) were used. Detection of MACC1 and c-Met was carried out with 3-amino-9-ethylcarbozole (AEC; Zhongshan Bio, China) and diaminobenzidine (DAB; R and D Systems, United States), respectively. Positive staining was indicated by a prominent brownish or red pigmentation. In each case, a negative control was prepared using phosphate buffered saline as the first antibody to ensure the specificity of immunostaining.
The extent of positive staining for MACC1 was scored as follows[15]: 0, ≤ 10%; 1, > 10%-25%; 2, > 25%-50%; 3, > 50%-75%; and 4, > 75%. The intensity of the special staining was scored as follows: 0, negative; 1+, weak; 2+, moderate; and 3+, strong. The final score was obtained by multiplying the extent scores and intensity scores, which produced values in a range from 0 to 12. Scores from 9-12 were defined as a strong staining pattern (++), scores from 0-4 were defined as negative expression (-), and scores from 6-8 were defined as an intermediate staining pattern (+). All the staining was evaluated and characterized by two independent pathologists.
Hepatitis B surface antigen (HBsAg), anti-HBs, HBeAg, anti-HBe and anti-HBc were detected using a commercially-available kit (Roche Diagnostics, United States) and electrochemiluminescence immunoassay analyzers (E170; Modular Analytics, Roche Diagnostics). HBV DNA was extracted from 200 μL of plasma sample from each study participant using a High Pure Viral Nucleic Acid Kit (Roche Diagnostics Applied Science, Germany) and following the manufacturer’s instructions. The viral titer and genotype of HBV were determined by a real-time PCR-based method that used fluorescent hybridization probes and a LightCycler PCR machine (Roche Diagnostics). This method consisted of two steps that were carried out in a single tube: the first step used real-time PCR to quantify the viral DNA and the second step used melting curve analysis of the final PCR product to genotype the virus. Details of the design and experimental conditions of this assay are available from the manufacturer. This assay showed a broad linear distribution for HBV titers that ranged from 102 to 1011 copies/mL, with a lower detection limit of 1-5 × 102 copies/mL.
The primary endpoint of the study was tumor-free survival (TFS) and the secondary endpoint included overall survival (OS) and follow-up for over 48 mo. TFS was calculated from the date of resection to the date when tumor recurrence was diagnosed. OS was calculated from the date of commencement of resection to the date of death or last follow-up[16]. All statistical analyses were performed with SPSS version 16.0 software. Continuous data were expressed as median and range. A comparison between the groups was performed using the χ2 test. Survival rates were estimated by the Kaplan-Meier method and compared by the log rank test. The Cox proportional hazards model was used to determine the independent factors on survival and recurrence, based on the variables selected in univariate analysis. P < 0.05 was considered statistically significant.
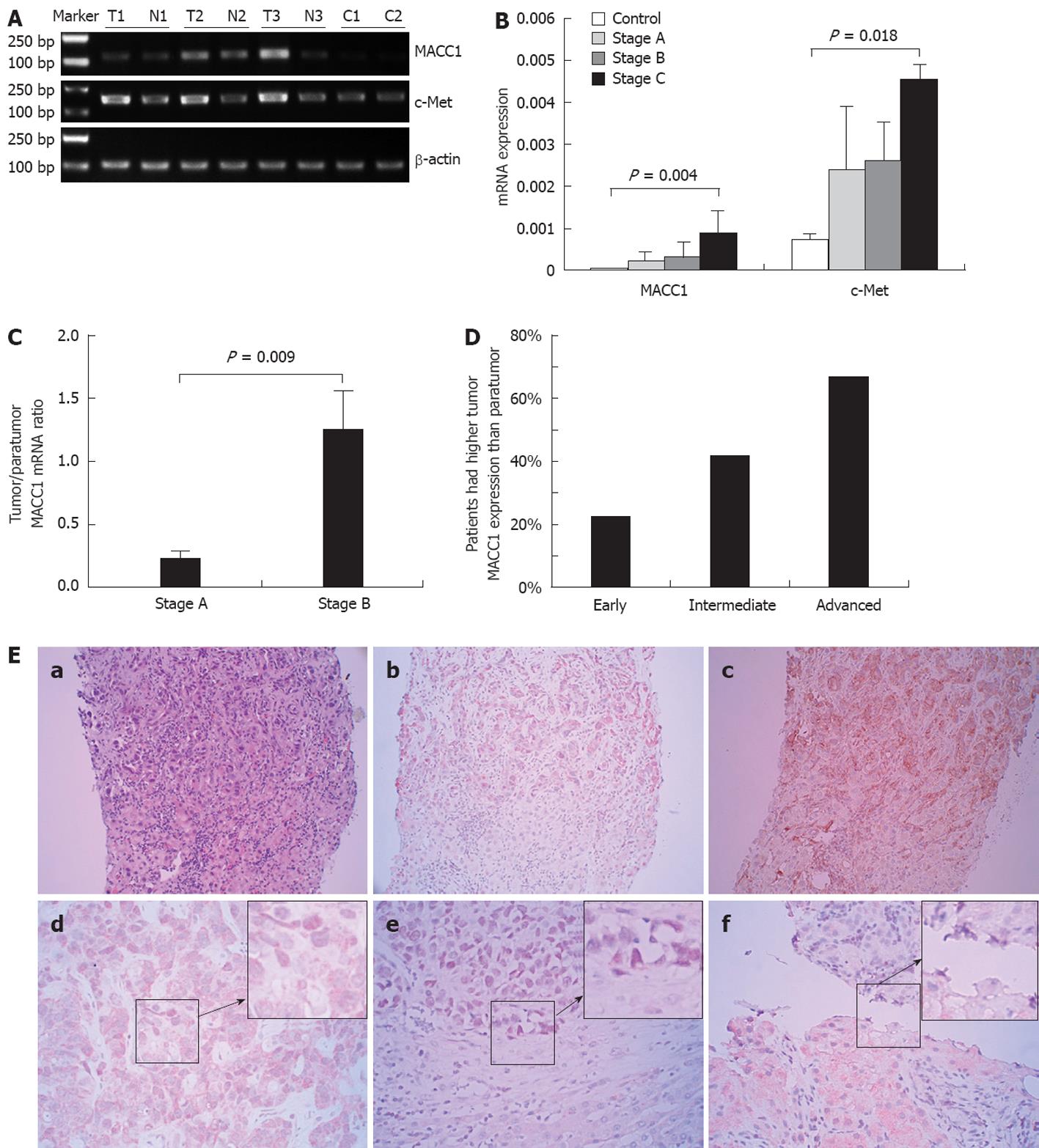
We analyzed the MACC1 mRNA levels in surgically-resected samples from 234 patients at BCLC stage A or stage B and in biopsied tumor tissues from 120 patients at BCLC stage C. MACC1 mRNA in tumor tissues was found to be increased gradually with the stage of HCC progression (Figure 1A and B). The intratumoral MACC1 mRNA levels detected in samples from HCC stage A (0.002281 ± 0.001972), B (0.003031 ± 0.003451) and C (0.009015 ± 0.004972) were about 3-, 4- and 14-fold higher than that in normal liver tissues (0.000592 ± 0.0000451), respectively. We next performed a paired comparison of gene expression for the 234 patients at stage A and stage B, for which we had matched tumor tissues and adjacent non-tumor liver tissues. The ratio of MACC1 mRNA in cancerous tissue relative to that of the matched paratumors (the T:N ratio) was about 5.4-fold higher in the stage B group than in the stage A group (1.25 ± 0.3 vs 0.23 ± 0.05, P = 0.009; Figure 1C). Thus, these data indicated that the MACC1 mRNA level in HCC tumors was associated with tumor progression.
We next determined the protein levels of MACC1 in tumor and paratumor tissues by analyzing immunohistochemistry scores. MACC1 protein levels were found to be significantly higher in malignant tissues than in paratumor tissues or normal liver tissues (both, P < 0.001). Compared with the corresponding peritumor tissue or normal liver tissues, tumors from 30 of 138 (22%) patients at stage A, 40 of 96 (41.6%) at stage B, and 80 of 120 (67%) at stage C displayed increased MACC1 expression (Figure 1D). Tumor cells demonstrated mild to strong positive MACC1 cytoplasmic staining (++) and apparent nuclear signals in some cases (Figure 1E).
Patients with HCC (n = 354) were divided into two groups according to the median intratumoral MACC1 mRNA levels. The first group was composed of low intratumoral MACC1 mRNA (< 0.006732; range: 0.000050-0.036147) and the second of high intratumoral MACC1 mRNA (≥ 0.006732; range: 0.000050-0.036147). Following comparative analysis of these two groups, intratumoral MACC1 mRNA level was found to be associated with HCC clinical staging, age, portal vein invasion and tumor differentiation. However, no significant correlation was found between the intratumoral MACC1 mRNA level and gender, lesion number, α-fetoprotein level or Child-Pugh class (Table 2).
| Variable | Cases (n = 354) | MACC1 mRNA high expression group | MACC1 mRNA low expression group | P |
| Gender | 0.214 | |||
| Male | 306 | 157 (88.7) | 149 (84.2) | |
| Female | 48 | 20 (11.3) | 28 (15.8) | |
| Age (yr) | < 0.001 | |||
| ≥ 55 | 185 | 70 (39.5) | 115 (65.0) | |
| < 55 | 169 | 107 (60.5) | 62 (35.0) | |
| Tumor size (cm) | 0.087 | |||
| ≥ 3 | 196 | 90 (50.8) | 106 (59.9) | |
| < 3 | 158 | 87 (49.2) | 71 (40.1) | |
| Tumor thrombus | 0.007 | |||
| Yes | 120 | 72 (40.7) | 48 (27.1) | |
| No | 234 | 105 (59.3) | 129 (72.9) | |
| Tumor number | 0.356 | |||
| Single | 246 | 119 (67.2) | 127 (71.8) | |
| Multinodular | 108 | 58 (32.8) | 50 (28.2) | |
| Stage | 0.007 | |||
| Early-middle | 234 | 105 (59.3) | 129 (72.9) | |
| Advanced | 120 | 72 (40.7) | 48 (27.1) | |
| Differentiation | < 0.001 | |||
| High | 92 | 21 (11.9) | 71 (40.1) | |
| Moderate | 154 | 92 (52.0) | 62 (35.0) | |
| Low | 108 | 64 (36.1) | 44 (24.9) | |
| AFP | 0.056 | |||
| ≤ 400 | 184 | 83 (46.9) | 101 (57.1) | |
| > 400 | 170 | 94 (53.1) | 76 (42.9) | |
| HBeAg | 0.190 | |||
| Positive | 136 | 62 (35.0) | 74 (41.8) | |
| Negative | 218 | 115 (65.0) | 103 (58.2) | |
| HBV DNA | 0.159 | |||
| Positive | 211 | 99 (55.9) | 112 (63.3) | |
| Negative | 143 | 78 (44.1) | 65 (36.7) | |
| Child-Pugh | 0.326 | |||
| A | 217 | 104 (58.8) | 113 (63.8) | |
| B | 137 | 73 (41.2) | 64 (36.2) |
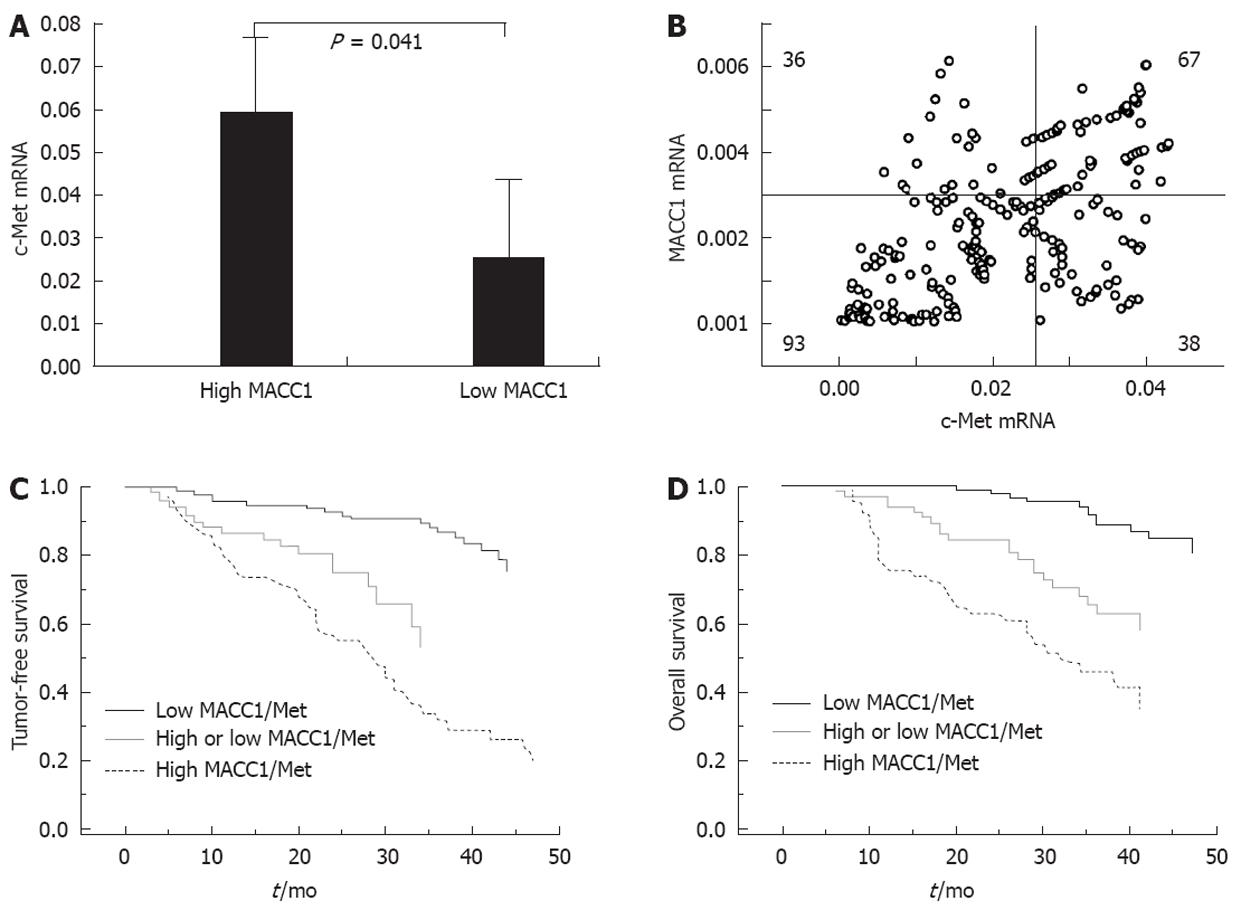
c-Met is a well-known proto-oncogene and transcriptional target of MACC1. We next investigated whether overexpression of MACC1 corresponded to increased transcription of c-Met in HCC tissues. We found that intratumoral c-Met mRNA levels were consistent with the extent of MACC1 expression and were up-regulated in conjunction with tumor progression of HCC (P < 0.01; Figure 1A and B). In the 234 HCC patients with stage A and stage B, the expression of c-Met was increased in the 105 patients with high MACC1 expression, but decreased in the 129 patients with low MACC1 expression (0.058561 ± 0.017539 vs 0.024734 ± 0.018754, P = 0.041; Figure 2A). Among these 234 patients, 67 (28.6%) displayed elevated expressions of both MACC1 and c-Met, 93 (39.7%) had low expressions of both MACC1 and c-Met, and 38 (16.2%) had a high expression of MACC1 but a low expression of c-Met, while 36 (15.4%) had a high expression of c-Met but low expression of MACC1. MACC1 mRNA level was closely correlated with the corresponding intratumoral c-Met mRNA expression (r = 0.360, P < 0.001) (Figure 2B).
Further analysis of the 234 patients found that HCC patients with both MACC1 and c-Met high intratumoral co-expression had a median OS of 32 mo [95% confidence interval (CI): 25-41]. In contrast, those with both MACC1 and c-Met low intratumoral expression had a median OS of 48 mo (log-rank, P < 0.001; Figure 2D). Likewise, the patients with both MACC1 and c-Met high intratumoral expression had significantly shorter median TFS (28 mo, 95% CI: 22-33 mo) than those with both MACC1 and c-Met low expression (48 mo, log-rank P < 0.001; Figure 2C).
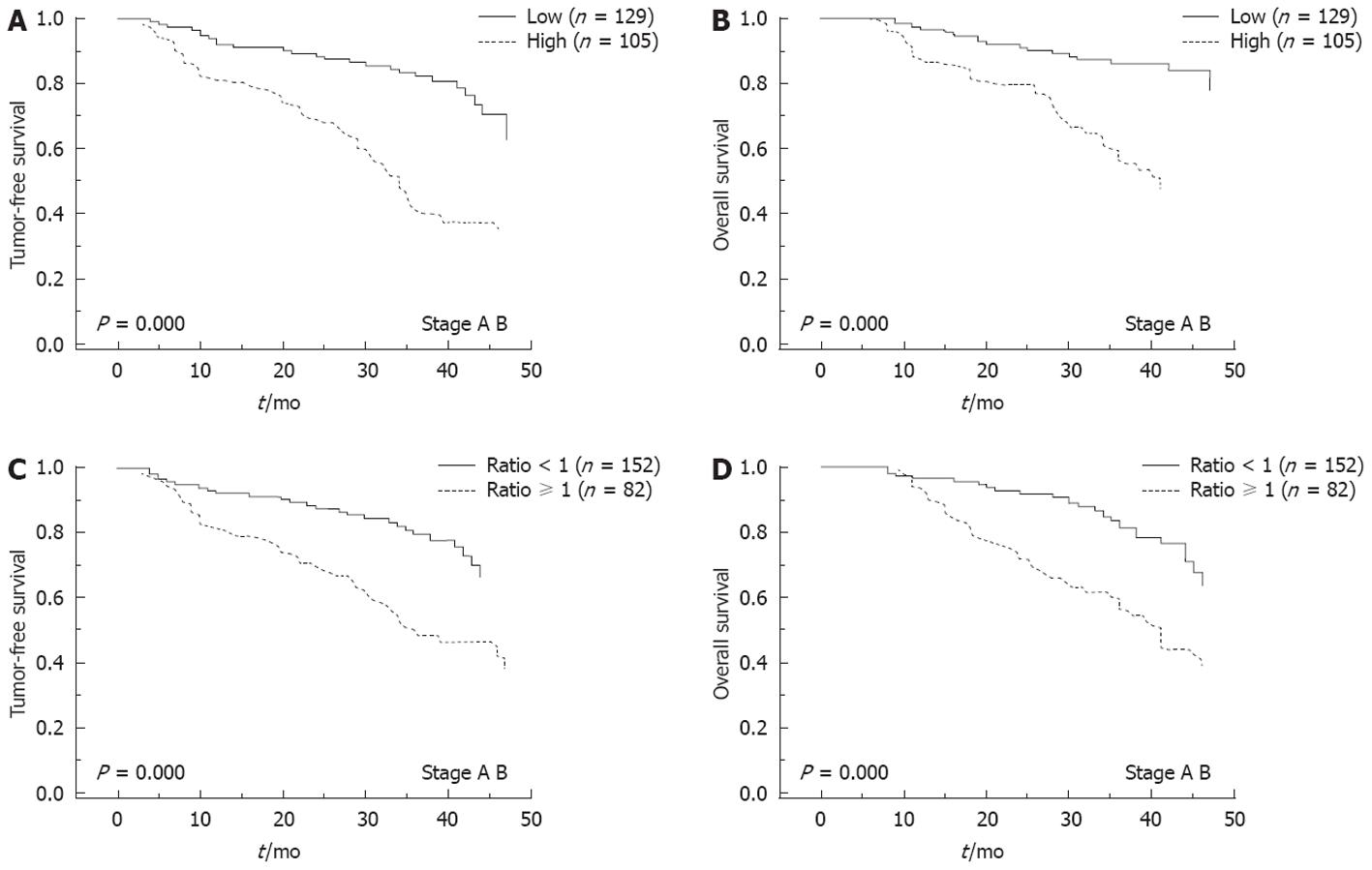
We followed up the 234 patients (stage A and stage B) after resection for a median of 30 mo (range: 6-48 mo). Seventy-nine of the 105 HCC patients (75.2%) with high intratumoral MACC1 mRNA levels experienced recurrent tumors, and 34 cases had extrahepatic metastasis. The 1-, 2- and 3-year recurrence-free survival rates were 81%, 67% and 39%, respectively. In contrast, only 41 of the 129 (31.8%) patients with low intratumoral MACC1 expression experienced recurrence, and 12 of those had extrahepatic metastasis. The 1-, 2- and 3-year TFS was 92%, 88% and 82%, respectively. Compared to those with low intratumoral MACC1 expression, patients with high intratumoral MACC1 had signicantly high rate of recurrence and extrahepatic metastasis (both, P < 0.001). Generally, the HCC patients with high intratumoral MACC1 mRNA levels had a significantly shorter median TFS (34 mo, 95% CI: 30-37 mo) than those with low intratumoral MACC1 mRNA levels (48.0 mo, log-rank P < 0.001; Figure 3A). In addition, post-resected patients with low intratumoral MACC1 expression had a median OS of 48 mo, while those with high intratumoral MACC1 expression had 40 mo (95% CI: 34-45 and log-rank P < 0.01; Figure 3B). A total of 152 patients with a ratio of T:N MACC1 expression < 1 had a median TFS of 48 mo, compared to 36 mo (95% CI: 25-47) for the 82 patients who had a ratio ≥ 1 (log-rank, P < 0.001; Figure 3C). Patients with a ratio of T:N MACC1 expression < 1 had a median OS of 48.0 mo, compared to 41 mo (95% CI: 36-45) for those with a ratio of T:N MACC1 expression ≥ 1 (log-rank, P < 0.001; Figure 3D).
Univariate statistical analysis showed that the TFS was associated with intratumoral MACC1 expression, and recurrence-free survival was related to tumor number, tumor differentiation, MACC1 expression, and co-expression of MACC1 and c-Met. The median OS was associated with Child-Pugh class, score from Eastern Cooperative Oncology Group performance status scale (ECOG PS), tumor differentiation, MACC1 mRNA levels, co-expression of MACC1 and c-Met, tumor number, and tumor size. Further multivariate analysis using the Cox hazards model revealed that a high MACC1 expression or co-expression with c-Met was an independent poor prognostic factor for TFS and OS. The combined expression of both MACC1 and c-Met increased these prognostic values, as compared to MACC1 overexpression alone (Table 3).
| Variables | TFS | OS | ||||||
| Univariate P | Multivariate | Univariate P | Multivariate | |||||
| Hazard ratios | 95% Cl | P | Hazard ratios | 95% Cl | P | |||
| Child-Pugh (A/B) | 0.184 | NA | NA | NA | 0.034 | 1.342 | 1.016-1.747 | 0.041 |
| Tumor differentiation (high/intermediate/low) | 0.037 | 1.213 | 0.743-1.980 | 0.441 | 0.038 | 1.133 | 0.683-1.679 | 0.624 |
| Tumor number (≥ 2/1) | 0.021 | 1.012 | 0.675-1.517 | 0.354 | 0.040 | 1.107 | 0.732-1.575 | 0.630 |
| ECOG PS (0/1/2) | 0.078 | NA | NA | NA | 0.045 | 1.079 | 0.893-1.530 | 0.679 |
| MACC1 (low/high) | < 0.001 | 1.489 | 1.071-1.801 | 0.013 | < 0.001 | 1.508 | 1.079-1.835 | 0.012 |
| MACC1 and c-Met expression (both/one) | < 0.001 | 1.929 | 1.207-3.083 | 0.006 | < 0.001 | 1.539 | 1.172-2.208 | 0.010 |
Consistent with the multifactorial aetiology of HCC and the long latent period of tumor formation, a large variety of cancer genes are involved in the multistep process of human hepatocarcinogenesis[3,4]. In order to identify suitable prognostic markers and therapeutic targets, it is essential to analyze gene expression and proteomic changes by evaluating a large series of HCC patients and samples at different disease stages. Moreover, since metastasis or recurrence is the major cause of death of postoperative HCC patients, early identification of subjects at high-risk for either of these processes is necessary to improve OS rates. The clinical factors related to tumor invasiveness, such as tumor size, number, histological type and vessel invasion, are considered the most related to risk for recurrence and the most useful for prediction of HCC patient outcome[17-19]. Molecular biology studies have identified many biological factors that may act as potential tumor prognostic markers. The fact that HCC metastasis is a multistep process involving many factors[20-22] has led to attempts to develop a panel of multiple biomarkers that will facilitate tumor diagnosis and prediction of tumor metastasis and recurrence.
MACC1 was recently identified as being involved in metastasis of colon cancers, presumably by up-regulating c-Met transcription. Thus, to elucidate the MACC1-related mechanism of HCC and identify potential targets for molecular-based therapies we investigated the expression of MACC1 and c-Met in HBV-induced HCC.
We determined that intratumoral MACC1 expression was significantly up-regulated in most of the late stage HCC tissues examined. We further found that MACC1 overexpression was associated with higher c-Met expression in HCC and the intratumoral MACC1 mRNA level alone or in combination with that of c-Met can serve as an independent predictive factor for recurrence and survival of postoperative HCC patients.
We statistically analyzed the correlation of intratumoral MACC1 mRNA levels and clinical parameters of HCC patients. High intratumoral MACC1 mRNA levels were significantly associated with clinical stage, age, vessel invasion, and tumor differentiation. MACC1 mRNA levels were found to gradually increase with the progression of HCC, especially at the advanced HCC stage, and this process was accompanied by invasion of the portal vein. Shirahata et al[23] also showed that MACC1 expression was significantly correlated with vascular invasion, as it was in our study. Furthermore, intratumoral MACC1 protein was localized mainly in the cell cytoplasm, where the levels increased from low to robust in conjunction with tumor progression, indicating that MACC1 may represent an effective biomarker of tumor progression. Previous studies in colon cancers had also found that intratumoral MACC1 was up-regulated, as compared to levels detected in matched peritumoral or normal colon mucosa, regardless of tumor stage classification[5]. In our study of hepatic cancer, we found that the paratumor livers in some HCC patients had a relatively strong expression of MACC1; this was especially the case for those patients with TFS shorter than 6 mo after resection, implying that the pathogenesis of HCC and colon cancers is likely distinct. However, considering that all of the patients examined in our study had a background of chronic HBV infection and liver cirrhosis, it is possible that the above-mentioned difference is due to the chronic hepatitis B infection.
Based on our immunohistochemistry data, the overexpression rates of MACC1 in HCC ranged from 22% to 67% in patients at different stages. MACC1 staining occurred mainly in the cytoplasm of non-cancerous or cancerous cells, but some cancerous cells showed significantly strong MACC1 staining in the nucleus. Studies in colon cancer have also identified MACC1 in the nuclear compartment of cancerous cells. It has been theorized that nuclear localization of MACC1 in conjunction with high c-Met levels contribute to the later development of distant metastases[5]. The actual clinical significance of the nuclear translocation of MACC1 in HCC requires further investigation.
It has been well documented that MACC1 binds to the c-Met promoter, and this transcription regulation event is crucial for tumor metastasis as induced by HGF/c-Met signaling[9]. A regulatory feedback mechanism exists in that HGF is able to promote the translocation of MACC1 to the nucleus, where MACC1 controls the promoter activity and hence expression of c-Met, thereby regulating c-Met-mediated signaling[6]. Our data showed that c-Met mRNA in intratumoral tissues was significantly higher than that in peritumoral tissues. Co-expression analysis showed a high consistency of MACC1 and c-Met mRNA in HCC tumors. Further investigation is required, however, to confirm whether MACC1 directly drives c-Met expression in HCC.
Previous studies on primary colon cancers indicated that the negative and positive predictions of MACC1 mRNA levels for distant metastases were 80% and 74%, respectively[5,24]. MACC1 can induce migration, invasion and proliferation of cultured cells[5], and can promote metastasis of tumor cells into liver and lung in various xenograft models. A current study in lung adenocarcinoma demonstrated that MACC1 overexpression was associated with postoperative recurrence[25]. In the present study, follow-up of 234 stage A and stage B HCC patients who received curative therapy revealed that a high intratumoral MACC1 expression level is correlated with a high rate of recurrence and extrahepatic metastasis. The median TFS and OS were much shorter in patients with high expression of both MACC1 and c-Met. High expression of intratumoral MACC1 was predictive of a poor outcome of HBV-related HCC, but when combined with high c-Met expression the predictive value for recurrence and metastasis increased.
Collectively, our data showed that intratumoral MACC1 expression is closely associated with tumor progression in HBV-induced HCC. Furthermore, elevated expression of MACC1 was statistically associated with poor outcome of these patients, suggesting that MACC1 is a novel predictor for recurrence and metastasis of postoperative HCC patients.
The authors wish to thank the numerous hospital staff who conducted the baseline and follow-up surveys. We would also like to express our appreciation to Zhi-Wei Li for collecting various patient samples, Jing-Min Zhao for his enthusiastic cooperation in the pathological analysis, and Song-Shan Wang for his technical assistance in immunohistochemical staining. We thank Medjaden Bioscience Limited for assisting in the preparation of this manuscript.
Due to metastasis or recurrence is the major cause of death of postoperative hepatocellular carcinoma (HCC) patients, early identification of subjects at high-risk for either of these processes is necessary to improve overall survival rates. It has been known that hepatocarcinogenesis is a complex process associated with the accumulation of multiple genetic and epigenetic changes during the initiation, progression and maturation, and molecular biology studies have identified many biological factors that may act as potential tumor prognostic markers, in the hope of developing effective preventative measures and improved treatment strategies.
The metastasis-associated in colon cancer 1 (MACC1) gene was identified by a genome-wide screen of human colon cancer samples, and its expression was closely related to the metastasis of colon cancers. Further studies have revealed that MACC1-induced tumorigenesis is correlated with enhanced hepatocyte growth factor/c-Met signaling.
The clinical significance of MACC1 overexpression in HCC and of the correlation between MACC1 and the c-Met signaling remain unknown. In the present study, authors sought to determine the expression levels of MACC1 in HBV-related HCC at different disease stages and analyze its correlation with clinical outcome. In addition, the authors evaluated the related levels of its transcriptional target, c-Met. The research indicated that MACC1 expression levels represent an effective prognostic factor for HBV-related HCC patients who undergo hepatectomy. The data showed that intratumoral MACC1 expression is closely associated with tumor progression in HBV-induced HCC. Furthermore, elevated expression of MACC1 was statistically associated with poor outcome of these patients.
The study results implied that the MACC1 is a novel predictor for recurrence and metastasis of postoperative HCC patients.
The MACC1 was recently identified as being involved in metastasis of colon cancers, presumably by up-regulating c-Met transcription.
The research is very important and the result is exciting and there is some clinical value, that is, intratumoral MACC1 expression may serve as a biomarker to predict recurrence or metastasis of postoperative HCC. The manuscript has a certain readability.
Peer reviewer: Shun-Fa Yang, PhD, Associate Professor, Institute of Medicine, Chung Shan Medical University, No. 110, Sec.1 Chien-Kuo N. Road, Taichung, 402, Taiwan, China
S- Editor Shi ZF L- Editor A E- Editor Zheng XM
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [PubMed] |
| 3. | Laurent-Puig P, Zucman-Rossi J. Genetics of hepatocellular tumors. Oncogene. 2006;25:3778-3786. [PubMed] |
| 4. | Lee JS, Thorgeirsson SS. Comparative and integrative functional genomics of HCC. Oncogene. 2006;25:3801-3809. [PubMed] |
| 5. | Stein U, Walther W, Arlt F, Schwabe H, Smith J, Fichtner I, Birchmeier W, Schlag PM. MACC1, a newly identified key regulator of HGF-MET signaling, predicts colon cancer metastasis. Nat Med. 2009;15:59-67. [PubMed] |
| 6. | Stein U, Smith J, Walther W, Arlt F. MACC1 controls Met: what a difference an Sp1 site makes. Cell Cycle. 2009;8:2467-2469. [PubMed] |
| 7. | Arlt F, Stein U. Colon cancer metastasis: MACC1 and Met as metastatic pacemakers. Int J Biochem Cell Biol. 2009;41:2356-2359. [PubMed] |
| 8. | Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915-925. [PubMed] |
| 9. | Boccaccio C, Comoglio PM. Invasive growth: a MET-driven genetic programme for cancer and stem cells. Nat Rev Cancer. 2006;6:637-645. [PubMed] |
| 10. | Stein U, Dahlmann M, Walther W. MACC1 - more than metastasis? Facts and predictions about a novel gene. J Mol Med (Berl). 2010;88:11-18. [PubMed] |
| 11. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [PubMed] |
| 12. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [PubMed] |
| 13. | Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101-1108. [PubMed] |
| 14. | Wang C, Lu Y, Chen Y, Feng Y, An L, Wang X, Su S, Bai W, Zhou L, Yang Y. Prognostic factors and recurrence of hepatitis B-related hepatocellular carcinoma after argon-helium cryoablation: a prospective study. Clin Exp Metastasis. 2009;26:839-848. [PubMed] |
| 15. | Wang ZL, Liang P, Dong BW, Yu XL, Yu de J. Prognostic factors and recurrence of small hepatocellular carcinoma after hepatic resection or microwave ablation: a retrospective study. J Gastrointest Surg. 2008;12:327-337. [PubMed] |
| 16. | Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M, Talwalkar J. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698-711. [PubMed] |
| 17. | Lau H, Fan ST, Ng IO, Wong J. Long term prognosis after hepatectomy for hepatocellular carcinoma: a survival analysis of 204 consecutive patients. Cancer. 1998;83:2302-2311. [PubMed] |
| 18. | Takenaka K, Kawahara N, Yamamoto K, Kajiyama K, Maeda T, Itasaka H, Shirabe K, Nishizaki T, Yanaga K, Sugimachi K. Results of 280 liver resections for hepatocellular carcinoma. Arch Surg. 1996;131:71-76. [PubMed] |
| 19. | Livraghi T, Bolondi L, Buscarini L, Cottone M, Mazziotti A, Morabito A, Torzilli G. No treatment, resection and ethanol injection in hepatocellular carcinoma: a retrospective analysis of survival in 391 patients with cirrhosis. Italian Cooperative HCC Study Group. J Hepatol. 1995;22:522-526. [PubMed] |
| 20. | Zhi H, Zhan J, Deng QL, Huang ZM. [Postoperative detection of AFP mRNA in the peripheral blood of hepatic cellular carcinoma patients and its correlation with recurrence]. Zhonghua Zhongliu Zazhi. 2007;29:112-115. [PubMed] |
| 21. | Zheng Q, Tang ZY, Xue Q, Shi DR, Song HY, Tang HB. Invasion and metastasis of hepatocellular carcinoma in relation to urokinase-type plasminogen activator, its receptor and inhibitor. J Cancer Res Clin Oncol. 2000;126:641-646. [PubMed] |
| 22. | Kamel L, Nessim I, Abd-el-Hady A, Ghali A, Ismail A. Assessment of the clinical significance of serum vascular endothelial growth factor and matrix metalloproteinase-9 in patients with hepatocellular carcinoma. J Egypt Soc Parasitol. 2005;35:875-890. [PubMed] |
| 23. | Shirahata A, Fan W, Sakuraba K, Yokomizo K, Goto T, Mizukami H, Saito M, Ishibashi K, Kigawa G, Nemoto H. MACC 1 as a marker for vascular invasive hepatocellular carcinoma. Anticancer Res. 2011;31:777-780. [PubMed] |
| 24. | Boardman LA. Overexpression of MACC1 leads to downstream activation of HGF/MET and potentiates metastasis and recurrence of colorectal cancer. Genome Med. 2009;1:36. [PubMed] |
| 25. | Shimokawa H, Uramoto H, Onitsuka T, Chundong G, Hanagiri T, Oyama T, Yasumoto K. Overexpression of MACC1 mRNA in lung adenocarcinoma is associated with postoperative recurrence. J Thorac Cardiovasc Surg. 2011;141:895-898. [PubMed] |