Copyright
©2012 Baishideng Publishing Group Co.
World J Gastroenterol. Apr 21, 2012; 18(15): 1781-1788
Published online Apr 21, 2012. doi: 10.3748/wjg.v18.i15.1781
Published online Apr 21, 2012. doi: 10.3748/wjg.v18.i15.1781
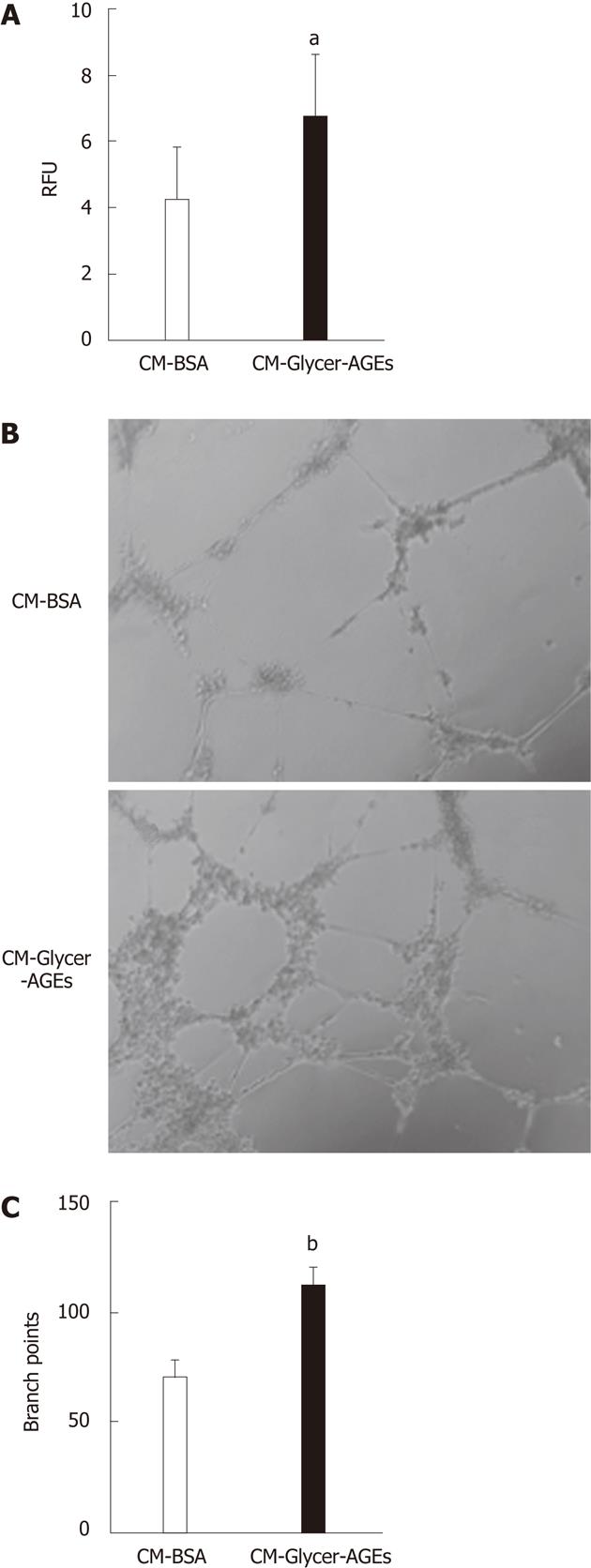
Figure 6 Effect of glyceraldehyde-derived advanced glycation end-products-treated CM on human umbilical vein endothelial cells angiogenesis.
A: The migratory capacity of human umbilical vein endothelial cells (HUVEC) was evaluated using the endothelial cell migration assay. Cells were incubated with CM-bovine serum albumin (BSA) or glyceraldehyde-derived advanced glycation end-products-treated CM (CM-Glycer-AGEs) for 22 h, and the number of migrating cells was assessed using a fluorescence microplate reader. RFU: relative fluorescence units (n = 8); B and C: The tube formation of HUVEC was evaluated using the endothelial cell tube formation assay (n = 5). Cells were incubated with CM-BSA or CM-Glycer-AGEs for 12 h and then photographed under a microscope (B); the number of branch points was counted (C). Magnification = 100 ×. The open and filled bars represent results for cells treated with CM-BSA and CM-Glycer-AGEs, respectively. Data are shown as the mean ± SD, aP < 0.05, bP < 0.01 vs CM-BSA.
- Citation: Takino J, Yamagishi S, Takeuchi M. Glycer-AGEs-RAGE signaling enhances the angiogenic potential of hepatocellular carcinoma by upregulating VEGF expression. World J Gastroenterol 2012; 18(15): 1781-1788
- URL: https://www.wjgnet.com/1007-9327/full/v18/i15/1781.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i15.1781