Published online Mar 28, 2012. doi: 10.3748/wjg.v18.i12.1385
Revised: August 1, 2011
Accepted: January 22, 2012
Published online: March 28, 2012
AIM: To evaluate the relationship between liver stiffness and duration of infection in blood transfusion-associated hepatitis C virus (HCV) patients with or without hepatocellular carcinoma (HCC).
METHODS: Between December 2006 and June 2008, a total of 524 transfusion-associated HCV-RNA positive patients with or without HCC were enrolled. Liver stiffness was obtained noninvasively by using Fibroscan (Echosens, Paris, France). The date of blood transfusion was obtained by interview. Duration of infection was derived from the interval between the date of blood transfusion and the date of liver stiffness measurement (LSM). Patients were stratified into four groups based on the duration of infection (17-29 years; 30-39 years; 40-49 years; and 50-70 years). The difference in liver stiffness between patients with and without HCC was assessed in each group. Multiple linear regression analysis was used to determine the factors associated with liver stiffness.
RESULTS: A total of 524 patients underwent LSM. Eight patients were excluded because of unsuccessful measurements. Thus 516 patients were included in the current analysis (225 with HCC and 291 without). The patients were 244 men and 272 women, with a mean age of 67.8 ± 9.5 years. The median liver stiffness was 14.3 kPa (25.8 in HCC group and 7.6 in non-HCC group). The patients who developed HCC in short duration of infection were male dominant, having lower platelet count, with a history of heavier alcohol consumption, showing higher liver stiffness, and receiving blood transfusion at an old age. Liver stiffness was positively correlated with duration of infection in patients without HCC (r = 0.132, P = 0.024) but not in patients with HCC (r = -0.103, P = 0.123). Liver stiffness was significantly higher in patients with HCC than in those without in each duration group (P < 0.0001). The factors significantly associated with high liver stiffness in multiple regression were age at blood transfusion (P < 0.0001), duration of infection (P = 0.0015), and heavy alcohol consumption (P = 0.043)
CONCLUSION: Although liver stiffness gradually increases over time, HCC develops in patients with high stiffness value regardless of the duration of infection.
- Citation: Masuzaki R, Tateishi R, Yoshida H, Arano T, Uchino K, Enooku K, Goto E, Nakagawa H, Asaoka Y, Kondo Y, Goto T, Ikeda H, Shiina S, Omata M, Koike K. Assessment of disease progression in patients with transfusion-associated chronic hepatitis C using transient elastography. World J Gastroenterol 2012; 18(12): 1385-1390
- URL: https://www.wjgnet.com/1007-9327/full/v18/i12/1385.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i12.1385
Hepatitis C virus (HCV) is a leading cause of chronic liver diseases, presenting serious public health problems worldwide[1,2]. HCV infection generally shows an asymptomatic onset and slow fibrosis progression, with cirrhosis developing after 20-50 years[3-7]. Progression of disease is known to depend on patients’ characteristics at the onset of infection[8-12]. Infection at old age, male gender and heavy alcohol consumption are reported to be independently associated with rapid disease progression.
The onset of HCV infection can be reliably estimated in transfusion-associated chronic hepatitis C patients, in contrast to repeating injecting-drug users. In Japan, about 40% of chronic hepatitis C patients and HCV-related HCC patients have a history of blood transfusion typically in 1950s and 1960s[13], when blood supply depended on paid blood donors. Not a few of the blood donors were also injecting-drug users, mainly methamphetamine, among whom HCV spread first after the end of World War II. Viral spread started to decline in Japan after commercial blood banks were entirely abolished in 1969[14].
Chronic hepatitis C with cirrhosis is a strong risk factor for hepatocellular carcinoma (HCC)[15,16]. It has been shown that the risk of HCC increases with the degree of liver fibrosis[17]. Until recently, however, the degree of liver fibrosis could be reliably assessed only with liver biopsy, an invasive procedure with the possibility of serious complications[18,19].
Liver stiffness, which can be noninvasively measured with transient elastography, has been recently reported to be well correlated with histologically assessed liver fibrosis stage[20]. We previously reported that liver stiffness is strongly associated with the risk of HCC[21,22]. The calculated stratum-specific likelihood ratio indicated that the post-test odds for the presence of HCC increase five-fold when liver stiffness is higher than 25 kPa and decrease to one-fifth when lower than 10 kPa. Furthermore, we have confirmed in a prospective study that liver stiffness is a significant risk factor for HCC development, together with male gender, clinical cirrhosis and serum albumin level. However, in those studies we did not fully consider the duration of HCV infection and the age at the onset of infection, which are indicated in several studies to be associated with disease progression.
The aim of this study is to evaluate the association between liver stiffness and the duration of infection in blood transfusion-associated hepatitis C patients with and without HCC, focusing on the risk of HCC development.
This study conformed to the ethical guideline of the 1975 Helsinki Declaration and the ethical guidelines for epidemiologic research designed by Japanese Ministry of Education, Culture, Sports, Science and Technology and Ministry of Health, Labor and Welfare. The study design was approved by the ethics committee of the authors’ institution. Between December 2006 and June 2008, a total of 1562 patients positive for HCV RNA visited the liver clinic of authors’ institution. Among these patients, those with a history of receiving blood transfusion were consecutively enrolled (229 with HCC and 295 without). We excluded from the present study those patients with concomitant hepatitis B virus surface antigen positivity, patients with uncontrollable ascites, patients on interferon therapy, and patients who received multiple blood transfusions.
Liver stiffness was obtained noninvasively by using Fibroscan (Echosens, Paris, France), a newly developed medical device based on elastometry. Liver stiffness measurement (LSM) was considered valid only when at least eight acquisitions were successful with a success rate of at least 60% and the ratio of inter-quartile range (IQR) to the median value was larger than 30%.
In patients with HCC, the cancer was diagnosed by dynamic computed tomography (CT), where intrahepatic nodules with hyperattenuation in the arterial phase with washout in the late phase were considered as definite HCC[23,24]. Histopathological diagnosis, using ultrasound-guided biopsy samples, was also performed when required. In patients without HCC, the cancer was ruled out by ultrasonography. No HCC was detected in the subsequent six-month follow-up period among these patients.
We determined the following parameters on the day of LSM: serum albumin and total bilirubin concentrations, serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels, prothrombin activity and platelet count. Serogrouping of HCV was assessed by enzyme-linked immunosorbent assay (ELISA) (Immucheck F-HCV Gr Kokusai; Sysmex, Kobe, Japan)[25]. Based on the prevalence of each HCV genotype in Japan, serogroup 1 was assumed to represent genotype 1b and serogroup 2 to represent genotype 2a or 2b.
The date of blood transfusion was obtained by interview. Duration of infection was derived from the interval between the date of blood transfusion and the date of LSM. The rate of liver stiffness progression was calculated as follows: present liver stiffness-minimal stiffness value in the cohort (kPa)/interval after blood transfusion (years).
Data were expressed as median and 25th-75th percentiles in parenthesis unless otherwise indicated. The categorical variables were compared by χ2 tests, whereas continuous variables were compared with unpaired Student’s t test (parametric) or Mann-Whitney U test (non-parametric). A P value < 0.05 on two-tailed test was considered significant.
The correlation between liver stiffness and the interval from blood transfusion was assessed by Spearman’s rank correlation. The duration of infection was arbitrarily stratified into 4 groups, 17-29 years; 30-39 years; 40-49 years; and 50-70 years. The difference in liver stiffness according to the presence of HCC was assessed in each group. Multiple linear regression analysis was used to determine the factors associated with liver stiffness. Processing and analysis were performed by using the S-plus Version 7 (TIBCO Software Inc., Palo Alto, CA, United States).
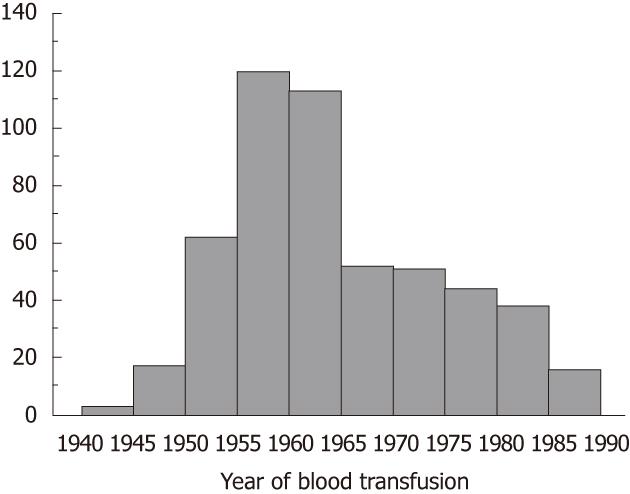
A total of 524 patients underwent LSM. Eight patients were excluded because of unsuccessful measurements, mostly due to obesity (four patients had IQR/median > 30% and four had a success rate lower than 60%). Thus 516 patients were included in the current analysis (225 with HCC and 291 without). Their characteristics at the time of LSM are summarized in Table 1. The patients were 244 men and 272 women, with a mean age of 67.8 ± 9.5 years. The median liver stiffness was 14.3 kPa (25.8 in HCC group and 7.6 in non-HCC group). Figure 1 shows the frequency distribution of the year of receiving blood transfusion among the subjects. A peak is noted around the year 1960.
| Characteristics | HCC | Non-HCC | P value |
| Overall patients | n = 225 | n = 291 | |
| Gender (M/F) | 126/99 | 118/173 | 0.0005 |
| Age (yr)1 | 71.2 (66.1-75.7) | 68.1 (58.7-72.4) | < 0.0001 |
| Platelet count (109/L)1 | 95 (74-133) | 161 (111-200) | < 0.0001 |
| ALT (IU/L)1 | 48 (34-68) | 42 (25-69) | 0.006 |
| Alcohol consumption > 50 g/d | 51 (22.7) | 28 (9.6) | < 0.0001 |
| Liver stiffness (kPa)1 | 25.8 (17.3-37.4) | 7.6 (5.6-13.9) | < 0.0001 |
| IQR (kPa)1 | 4.0 (2.5-6.0) | 1.6 (1.2-2.6) | < 0.0001 |
| Duration (17-29 yr) | n = 34 | n = 64 | |
| Gender (M/F) | 25/9 | 38/26 | 0.0028 |
| Age (yr)1 | 73.1 (65.7-77.1) | 59.7 (47.2-69.2) | 0.033 |
| Platelet count (109/L)1 | 95 (76-154) | 180 (116-229) | < 0.0001 |
| ALT (IU/L)1 | 51 (34-89) | 42 (22-77) | 0.2071 |
| Alcohol consumption > 50 g/d | 12 (35.3) | 9 (14.1) | 0.023 |
| Liver stiffness (kPa)1 | 26.1 (16.8-53.3) | 5.9 (4.9-12.1) | < 0.0001 |
| Duration (30-39 yr) | n = 40 | n = 59 | |
| Gender (M/F) | 16/24 | 23/36 | 0.9191 |
| Age (yr)1 | 72.0 (65.4-76.7) | 62.3 (55.7-68.6) | < 0.0001 |
| Platelet count (109/L)1 | 93 (68-120) | 151 (97-215) | < 0.0001 |
| ALT (IU/L)1 | 42 (33-65) | 48 (27-80) | 0.7591 |
| Alcohol consumption > 50 g/d | 6 (15) | 7 (11.9) | 0.7641 |
| Liver stiffness (kPa)1 | 28.7 (20.1-37.8) | 7.4 (5.7-13.8) | < 0.0001 |
| Duration (40-49 yr) | n = 101 | n = 127 | |
| Gender (M/F) | 58/43 | 51/76 | 0.0113 |
| Age (yr)1 | 69.2 (65.8-73.6) | 69.9 (65.7-72.7) | 0.8107 |
| Platelet count (109/L)1 | 97 (67-136) | 163 (112-195) | < 0.0001 |
| ALT (IU/L)1 | 48 (34-69) | 38 (23-64) | 0.0080 |
| Alcohol consumption > 50 g/d | 25 (24.8) | 8 (6.3) | 0.0001 |
| Liver stiffness (kPa)1 | 25.1 (17.5-37.4) | 8.2 (5.8-14.1) | < 0.0001 |
| Duration (50-70 yr) | n = 50 | n = 41 | |
| Gender (M/F) | 27/23 | 18/23 | 0.4016 |
| Age (yr)1 | 74.4 (70.0-78.1) | 73.7 (66.3-79.2) | 0.5658 |
| Platelet count (109/L)1 | 97 (81-141) | 147 (117-189) | 0.0001 |
| ALT (IU/L)1 | 52 (36-69) | 46 (32-63) | 0.1700 |
| Alcohol consumption > 50 g/d | 8 (16) | 4 (9.8) | 0.5363 |
| Liver stiffness (kPa)1 | 16.0 (8.0-36.3) | 7.9 (6.5-15.8) | < 0.0001 |
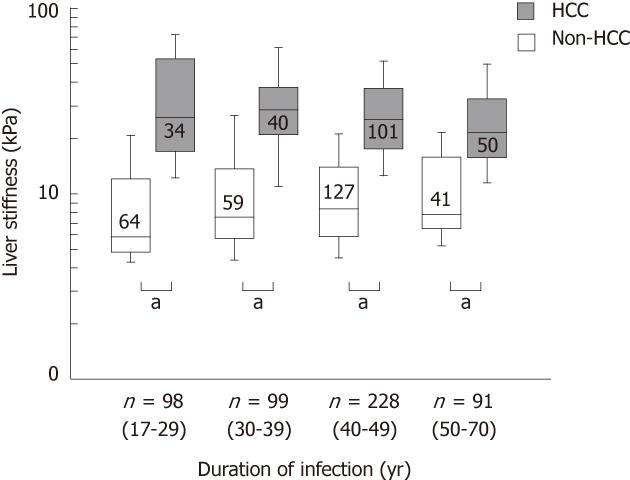
Characteristics of patients were compared between patients with and without HCC in each duration of infection group (Table 1). The patients who developed HCC in short duration of infection were male dominant, having low platelet count, with a history of heavier alcohol consumption, showing higher liver stiffness, and receiving blood transfusion at an older age.
The correlation between liver stiffness and duration of infection was significant in patients without HCC (r = 0.132, Z = 2.256, P = 0.024) but not in patients with HCC (r = -0.103, Z = -1.54, P = 0.123). When the duration of infection was stratified into 4 groups, 17-29 years; 30-39 years; 40-49 years; and 50-70 years, liver stiffness was higher in patients with HCC than in patients without in each group (P < 0.0001, Figure 2).
The relationship between present liver stiffness and patients’ characteristics, i.e., the age at blood transfusion, duration of infection, gender, and alcohol consumption (alcohol > 50 g/d) was analyzed with multiple linear regression analysis. The results showed that the age at blood transfusion was positively correlated with liver stiffness, with a coefficient of +0.336 per year for kPa, P < 0.0001, independently of the duration of infection (coefficient +0.272 per year for kPa, P = 0.0015). This suggests that fibrosis progression is more rapid when infection is acquired at older ages. Alcohol consumption was also significantly correlated with a positive coefficient (coefficient +4.183 for kPa, P = 0.043).
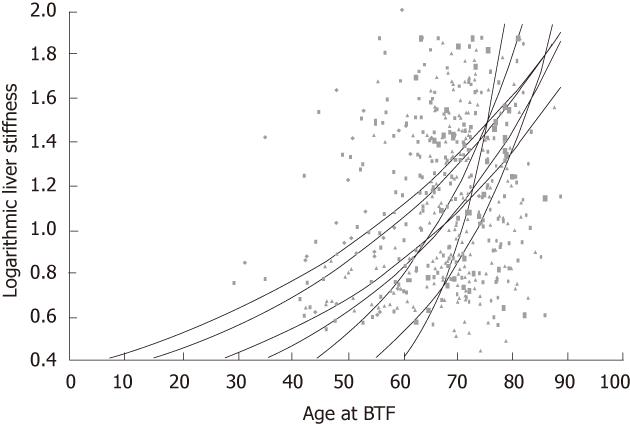
The progression of liver fibrosis, as represented by the increase in liver stiffness, must have been rapid in patients who have high liver stiffness in spite of short duration of HCV infection. We assumed that the liver stiffness was normal, that is, 2.9 kPa, when patients received blood transfusion. In Figure 3, the slopes represent the estimated increase rates of liver stiffness. In accordance with the results of multiple regression, the estimated increase rate was higher when patients received blood transfusion at older ages.
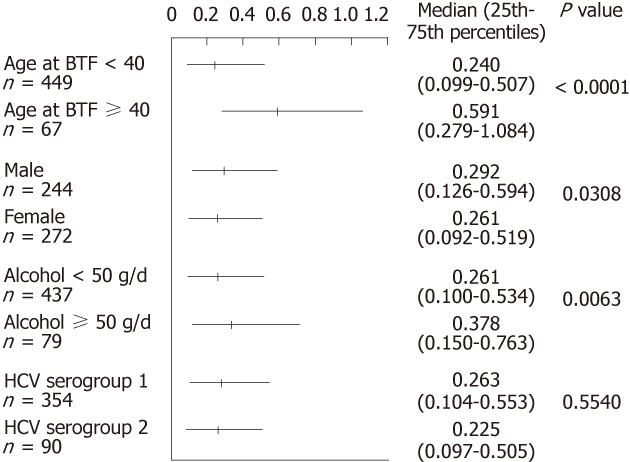
The progression rate of liver stiffness was assessed in subgroups according to three parameters (Figure 4). The progression rate was significantly higher in patients who were older than 40 at the time of blood transfusion (P < 0.0001), which is in accordance with the results of multiple regression. Heavy alcohol consumption (more than 50 g ethanol/d, P = 0.0308) and male gender (P = 0.0063) also showed significant difference by Mann-Whitney U test. There was no significant difference among HCV genotypes.
The natural history of chronic hepatitis C concerning liver fibrosis progression has been vigorously studied using liver biopsy specimens. The extent of liver fibrosis is usually evaluated as categorical stages. For example, METAVIR Score uses five stages, F0-F4, for fibrosis evaluation[26]. The fibrosis progression in hepatitis C patients, calculated by using paired liver biopsy, was reported to be 0.1-0.133 Unit per year[12,13]. Liver stiffness measured by transient elastography is now widely accepted as a surrogate marker of liver fibrosis[27]. Liver stiffness is expressed as a continuous variable in kPa unit. The cut-off for cirrhosis is reportedly 13-17 kPa, and the upper limit of measurement is currently 75 kPa. Thus LSM has a wider dynamic range than histological staging, and the rate of fibrosis progression may be more accurately analyzed with LSM.
In the present study, the increase rate of liver stiffness was positively correlated with the age at blood transfusion, as shown by the steeper slopes of approximation curves when patients received BTF at older ages. The cause of this phenomenon is not clear but age-related changes in immunity may be involved. If this is the case, the increase rate is likely to become higher in the same patient with age. Indeed, each approximation curve in the figure apparently becomes steeper with age, suggesting age-related acceleration. This is to be confirmed in future longitudinal studies.
LSM is useful not only as a surrogate of liver biopsy but also as a risk indicator of HCC development. Indeed, in the present study, liver stiffness is high in patients with HCC regardless of duration of infection. The patients who developed HCC with short duration of infection received blood transfusion at an older age and were older at the time of LSM, male dominant, and showed higher liver stiffness than patients without HCC with similar duration of infection. The difference between patients with and without HCC became smaller with longer duration of infection, as the average liver stiffness in patients with HCC became lower and that in patients without HCC became higher. We speculated that patients with high liver stiffness who received blood transfusion in the early period have already died of HCC or liver failure and were eliminated from the study population. Another possibility is that HCC may develop in patients with relatively low liver stiffness when infection has lasted a long time.
In the present study, the median increase in liver stiffness was calculated as 0.275 kPa per year. Using 13.01 kPa as a cut-off for cirrhosis[28], it will take around 40 years on average to develop cirrhosis, which is consistent with previous reports based on liver biopsy[29]. Admittedly, the present study is basically cross-sectional, and prospective longitudinal LSM will be obviously superior in understanding the natural course of liver fibrosis progression. However, the estimated average increase rate of liver stiffness indicates that such studies will require repeated LSM at an interval of at least five years.
Age at viral infection, alcohol consumption, and male gender were reported to be associated with accelerated fibrosis progression[8-11]. In the present study, we performed subgroup analysis and indeed found that blood transfusion at an age older than 40, male gender, and alcohol consumption more than 50 g ethanol/d were significantly associated with rapid increase in liver stiffness. There is consensus that heavy alcohol consumption is associated with fibrosis progression[30]. Alcohol, which by itself can cause liver disease and fibrosis, may affect liver stiffness and worsen fibrosis in hepatitis C[31]. We did not find a difference in liver stiffness increase rate between HCV genotypes 1 (mostly 1b) and 2 (2a/2b), although we could not evaluate genotypes 1a, 3 or 4.
This study has some limitations. First, since this is a cross-sectional study performed after LSM became available, patients with more rapid disease progression may have died and been excluded from the study. Second, because transfusion-associated HCV infection has been virtually eliminated in Japan since 1992, we could not include patients with shorter duration of infection. Lastly, we did not perform paired LSM but assumed that liver stiffness was normal at the time of infection. Longitudinal observation is now on-going but will take several years to obtain results.
In conclusion, although liver stiffness gradually increases over time from the onset of infection in general, HCC develops in patients with high liver stiffness regardless of the duration of infection. Patients who acquired HCV infection at older ages showed higher increase rate of liver stiffness and probably more rapid disease progression.
Liver stiffness, which can be noninvasively measured with transient elastography, has been recently reported to be well correlated with histologically assessed liver fibrosis stage.
This study evaluated the association between liver stiffness and the duration of infection in blood transfusion-associated hepatitis C patients with and without hepatocellular carcinoma (HCC), focusing on the risk of HCC development.
Liver stiffness is expressed as a continuous variable in kPa unit. The cut-off for cirrhosis is reportedly 13-17 kPa, and the upper limit of measurement is currently 75 kPa. Thus liver stiffness measurement (LSM) has a wider dynamic range than histological staging, and the rate of fibrosis progression may be more accurately analyzed with LSM.
Although liver stiffness gradually increases over time from the onset of infection in general, HCC develops in patients with high liver stiffness regardless of the duration of infection. Patients who acquired hepatitis C virus (HCV) infection at older ages showed higher increase rate of liver stiffness and probably more rapid disease progression.
Transient elastography (Fibro-Scan®; EchoSens, Paris, France) is a rapid, reliable and well-tolerated imaging technique for the assessment of liver fibrosis by measuring liver stiffness.
This is an interesting and timely study on liver stiffness in patients with transfusion associatated HCV. The authors show that HCC develops in patients with high liver stiffness regardless of the duration of infection. Patients who acquired HCV infection at older ages showed higher increase rate of liver stiffness. Co-exposure to alcohol is critical. The methodology is sound and the paper is well and clearly written.
Peer reviewer: Sebastian Mueller, MD, PhD, Professor of Medicine, Department of Internal Medicine, Salem Medical Center, and Center for Alcohol Research, University of Heidelberg, Zeppelinstraße 11-33, Heidelberg 69121, Germany
S- Editor Shi ZF L- Editor O’Neill M E- Editor Xiong L
| 1. | Strader DB, Wright T, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39:1147-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 1283] [Cited by in RCA: 1348] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 2. | Di Bisceglie AM. Hepatitis C. Lancet. 1998;351:351-355. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 266] [Cited by in RCA: 265] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 3. | Seeff LB. The history of the "natural history" of hepatitis C (1968-2009). Liver Int. 2009;29 Suppl 1:89-99. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in RCA: 164] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 4. | Alberti A, Chemello L, Benvegnù L. Natural history of hepatitis C. J Hepatol. 1999;31 Suppl 1:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 234] [Cited by in RCA: 235] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 5. | Kiyosawa K, Sodeyama T, Tanaka E, Gibo Y, Yoshizawa K, Nakano Y, Furuta S, Akahane Y, Nishioka K, Purcell RH. Interrelationship of blood transfusion, non-A, non-B hepatitis and hepatocellular carcinoma: analysis by detection of antibody to hepatitis C virus. Hepatology. 1990;12:671-675. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 927] [Cited by in RCA: 941] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 6. | Marcellin P, Asselah T, Boyer N. Fibrosis and disease progression in hepatitis C. Hepatology. 2002;36:S47-S56. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in RCA: 144] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Seeff LB, Hollinger FB, Alter HJ, Wright EC, Cain CM, Buskell ZJ, Ishak KG, Iber FL, Toro D, Samanta A. Long-term mortality and morbidity of transfusion-associated non-A, non-B, and type C hepatitis: A National Heart, Lung, and Blood Institute collaborative study. Hepatology. 2001;33:455-463. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in RCA: 243] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 8. | Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825-832. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 2199] [Cited by in RCA: 2142] [Article Influence: 76.5] [Reference Citation Analysis (0)] |
| 9. | Matsumura H, Moriyama M, Goto I, Tanaka N, Okubo H, Arakawa Y. Natural course of progression of liver fibrosis in Japanese patients with chronic liver disease type C--a study of 527 patients at one establishment. J Viral Hepat. 2000;7:268-275. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Hamada H, Yatsuhashi H, Yano K, Daikoku M, Arisawa K, Inoue O, Koga M, Nakata K, Eguchi K, Yano M. Impact of aging on the development of hepatocellular carcinoma in patients with posttransfusion chronic hepatitis C. Cancer. 2002;95:331-339. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Khan KN, Yatsuhashi H. Effect of alcohol consumption on the progression of hepatitis C virus infection and risk of hepatocellular carcinoma in Japanese patients. Alcohol Alcohol. 2000;35:286-295. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Minola E, Prati D, Suter F, Maggiolo F, Caprioli F, Sonzogni A, Fraquelli M, Paggi S, Conte D. Age at infection affects the long-term outcome of transfusion-associated chronic hepatitis C. Blood. 2002;99:4588-4591. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in RCA: 115] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Yoshizawa H, Tanaka J, Miyakawa Y. National prevention of hepatocellular carcinoma in Japan based on epidemiology of hepatitis C virus infection in the general population. Intervirology. 2006;49:7-17. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Tanaka Y, Hanada K, Mizokami M, Yeo AE, Shih JW, Gojobori T, Alter HJ. A comparison of the molecular clock of hepatitis C virus in the United States and Japan predicts that hepatocellular carcinoma incidence in the United States will increase over the next two decades. Proc Natl Acad Sci USA. 2002;99:15584-15589. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in RCA: 260] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 15. | Masuzaki R, Yoshida H, Tateishi R, Shiina S, Omata M. Hepatocellular carcinoma in viral hepatitis: improving standard therapy. Best Pract Res Clin Gastroenterol. 2008;22:1137-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Ikeda K, Saitoh S, Suzuki Y, Kobayashi M, Tsubota A, Koida I, Arase Y, Fukuda M, Chayama K, Murashima N. Disease progression and hepatocellular carcinogenesis in patients with chronic viral hepatitis: a prospective observation of 2215 patients. J Hepatol. 1998;28:930-938. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 316] [Cited by in RCA: 314] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 17. | Yoshida H, Shiratori Y, Moriyama M, Arakawa Y, Ide T, Sata M, Inoue O, Yano M, Tanaka M, Fujiyama S. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of Hepatocarcinogenesis by Interferon Therapy. Ann Intern Med. 1999;131:174-181. [PubMed] [Cited in This Article: ] |
| 18. | Dienstag JL. The role of liver biopsy in chronic hepatitis C. Hepatology. 2002;36:S152-S160. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in RCA: 96] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 1193] [Cited by in RCA: 1390] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 20. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 1796] [Cited by in RCA: 1832] [Article Influence: 91.6] [Reference Citation Analysis (0)] |
| 21. | Masuzaki R, Tateishi R, Yoshida H, Goto E, Sato T, Ohki T, Imamura J, Goto T, Kanai F, Kato N. Prospective risk assessment for hepatocellular carcinoma development in patients with chronic hepatitis C by transient elastography. Hepatology. 2009;49:1954-1961. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in RCA: 296] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 22. | Masuzaki R, Tateishi R, Yoshida H, Yoshida H, Sato S, Kato N, Kanai F, Sugioka Y, Ikeda H, Shiina S. Risk assessment of hepatocellular carcinoma in chronic hepatitis C patients by transient elastography. J Clin Gastroenterol. 2008;42:839-843. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Makuuchi M, Kokudo N, Arii S, Futagawa S, Kaneko S, Kawasaki S, Matsuyama Y, Okazaki M, Okita K, Omata M. Development of evidence-based clinical guidelines for the diagnosis and treatment of hepatocellular carcinoma in Japan. Hepatol Res. 2008;38:37-51. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in RCA: 232] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 24. | Kudo M, Okanoue T. Management of hepatocellular carcinoma in Japan: consensus-based clinical practice manual proposed by the Japan Society of Hepatology. Oncology. 2007;72 Suppl 1:2-15. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in RCA: 152] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 25. | Moriyama M, Matsumura H, Nirei K, Arakawa Y, Yamagami H, Ogawa M, Kaneko M, Matsuoka S, Amaki S, Tanaka N. Factors influencing treatment efficacy of 24-week combination therapy with interferon alpha-2b plus ribavirin for chronic hepatitis C. Dig Dis Sci. 2007;52:2418-2426. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 2860] [Cited by in RCA: 3049] [Article Influence: 105.1] [Reference Citation Analysis (0)] |
| 27. | Ghany MG, Kleiner DE, Alter H, Doo E, Khokar F, Promrat K, Herion D, Park Y, Liang TJ, Hoofnagle JH. Progression of fibrosis in chronic hepatitis C. Gastroenterology. 2003;124:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 299] [Cited by in RCA: 267] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 28. | Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, Herrmann E. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960-974. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 1046] [Cited by in RCA: 1055] [Article Influence: 62.1] [Reference Citation Analysis (1)] |
| 29. | Shiratori Y, Imazeki F, Moriyama M, Yano M, Arakawa Y, Yokosuka O, Kuroki T, Nishiguchi S, Sata M, Yamada G. Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med. 2000;132:517-524. [PubMed] [Cited in This Article: ] |
| 30. | EASL International Consensus Conference on Hepatitis C. Paris, 26-28, February 1999, Consensus Statement. European Association for the Study of the Liver. J Hepatol. 1999;30:956-961. [PubMed] [Cited in This Article: ] |
| 31. | Mueller S, Millonig G, Sarovska L, Friedrich S, Reimann FM, Pritsch M, Eisele S, Stickel F, Longerich T, Schirmacher P. Increased liver stiffness in alcoholic liver disease: differentiating fibrosis from steatohepatitis. World J Gastroenterol. 2010;16:966-972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: ] [Cited by in CrossRef: 160] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |