Published online Mar 7, 2011. doi: 10.3748/wjg.v17.i9.1095
Revised: December 21, 2010
Accepted: December 28, 2010
Published online: March 7, 2011
Natural and synthetic glucocorticoids (GCs) are widely employed in a number of inflammatory, autoimmune and neoplastic diseases, and, despite the introduction of novel therapies, remain the first-line treatment for inducing remission in moderate to severe active Crohn’s disease and ulcerative colitis. Despite their extensive therapeutic use and the proven effectiveness, considerable clinical evidence of wide inter-individual differences in GC efficacy among patients has been reported, in particular when these agents are used in inflammatory diseases. In recent years, a detailed knowledge of the GC mechanism of action and of the genetic variants affecting GC activity at the molecular level has arisen from several studies. GCs interact with their cytoplasmic receptor, and are able to repress inflammatory gene expression through several distinct mechanisms. The glucocorticoid receptor (GR) is therefore crucial for the effects of these agents: mutations in the GR gene (NR3C1, nuclear receptor subfamily 3, group C, member 1) are the primary cause of a rare, inherited form of GC resistance; in addition, several polymorphisms of this gene have been described and associated with GC response and toxicity. However, the GR is not self-standing in the cell and the receptor-mediated functions are the result of a complex interplay of GR and many other cellular partners. The latter comprise several chaperonins of the large cooperative hetero-oligomeric complex that binds the hormone-free GR in the cytosol, and several factors involved in the transcriptional machinery and chromatin remodeling, that are critical for the hormonal control of target genes transcription in the nucleus. Furthermore, variants in the principal effectors of GCs (e.g. cytokines and their regulators) have also to be taken into account for a comprehensive evaluation of the variability in GC response. Polymorphisms in genes involved in the transport and/or metabolism of these hormones have also been suggested as other possible candidates of interest that could play a role in the observed inter-individual differences in efficacy and toxicity. The best-characterized example is the drug efflux pump P-glycoprotein, a membrane transporter that extrudes GCs from cells, thereby lowering their intracellular concentration. This protein is encoded by the ABCB1/MDR1 gene; this gene presents different known polymorphic sites that can influence its expression and function. This editorial reviews the current knowledge on this topic and underlines the role of genetics in predicting GC clinical response. The ambitious goal of pharmacogenomic studies is to adapt therapies to a patient’s specific genetic background, thus improving on efficacy and safety rates.
- Citation: Iudicibus SD, Franca R, Martelossi S, Ventura A, Decorti G. Molecular mechanism of glucocorticoid resistance in inflammatory bowel disease. World J Gastroenterol 2011; 17(9): 1095-1108
- URL: https://www.wjgnet.com/1007-9327/full/v17/i9/1095.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i9.1095
Glucocorticoids (GCs) are a well-accepted therapy for inflammatory, autoimmune and proliferative diseases[1-3]. Despite the large clinical use, the benefits of these agents are often narrowed by inter-individual variability. Indeed, some patients show a poor or absent response, and in these subjects, GCs have to be employed in high doses. The clinical use of these compounds is associated with a number of serious complications, including osteoporosis, metabolic disease and increased risk of cardiovascular disease[4-6]; therefore, patients, and in particular those who respond poorly to these agents, are at high risk of side effects. In addition, besides being a problem for patients and a challenge to clinicians, inadequate GC therapy also represents a socio-economic matter because of the consequent considerable impact on health care costs[7,8].
GCs are effective inhibitors of cytokine secretion and T-cell activation, and are consequently largely employed in different inflammatory conditions, including inflammatory bowel disease (IBD). In these diseases, GC resistance or dependence is particularly frequent. Clinical reports in pediatric IBD patients have shown that up to 90% of subjects have a rapid improvement of symptoms when prednisone is given[9]. However, after 1 year, only 55% of early steroid-treated patients are still in remission and can be considered steroid-responsive. Around 38% of patients are not able to discontinue the therapy and experience an increase in disease activity when the dose is reduced (steroid-dependent); 7% of subjects are resistant and do not respond to GC therapy[9,10]. Among the adult IBD population, a prospective analysis has described the 1-year outcome in patients with Crohn’s disease (CD) treated with a first oral prednisolone course (40-60 mg/d) and tapering to a maintenance dose of 10-15 mg/d[11]. In this study, prolonged steroid response was obtained in 44% of patients, 36% of subjects were steroid dependent and 20% steroid resistant, and a high frequency of surgery was reported within 1 mo after steroid treatment. Similar results have been obtained in a retrospective American study: immediate outcomes for CD and ulcerative colitis (UC) respectively, were complete remission in 58% and 54%, partial remission in 26% and 30%, resistance in 16% of patients[12]. Outcome at 1 year showed a prolonged response in 32% of CD patients, GC dependence in 28%, and surgical intervention in 38%. In UC patients, 1-year outcomes were prolonged response in 49%, dependence in 22%, and surgery in 29% of subjects.
In 1999, a pivotal study by Hearing et al[13] demonstrated a poor in vitro response to GCs of circulating lymphocytes in UC patients who exhibited a reduced or absent clinical response to these agents. In vitro lymphocyte resistance to GCs has been demonstrated to correlate with clinical outcome in other inflammatory diseases such as asthma[14], systemic lupus erythematosus[15], rheumatoid arthritis[16] and renal allograph rejection[17]. A wide variation in lymphocyte steroid sensitivity is evident even in healthy individuals[18]. This suggests that the observed variability is a stable intrinsic property of an individual that becomes important when the subject has to be treated with GCs for an inflammatory disease[19], and is therefore likely to have a genetic basis.
Over the past years, significant advances have been made in understanding the molecular mechanisms by which GCs act and the basis of such inter-patient variability. This editorial describes the mechanisms of GC anti-inflammatory action and discusses the molecular and genetic basis for GC resistance in chronic inflammatory diseases, especially IBD.
Due to their high lipophilicity, exogenous GCs are widely bioavailable; these agents, as well as the endogenous compound cortisol, are transported in the blood predominantly bound to the corticosteroid-binding globulin and, to a lesser extent, to albumin[20]. Free GCs are able to diffuse passively across plasma membranes and interact specifically with a cytosolic receptor (GR), expressed in virtually all tissues. The GC receptor is a member of the nuclear receptor (NR) superfamily, which includes receptors for steroid hormones (e.g. corticosteroids, androgens, estrogens and progesterone), as well as other hydrophobic molecules (such as bile acids, vitamins A and D, retinoic acid and thyroid hormones). All these molecules induce their actions by the same molecular mechanism: at the basis of this mechanism stands the physical interaction between the lipophilic ligand and its own cytosolic/nuclear receptor that, in turn, activates a multistep signal transduction pathway to end up in specific genomic transcriptional effects[21]. Receptors belonging to the NR superfamily are highly homologous to each other and share structural features with a common modular domain organization: they present a transactivation domain at the N-terminal part (NTD), a central zinc finger DNA-binding domain (DBD) and a ligand-specific binding domain (LBD) at the C terminus.
The ligand-free GR is not self-standing in the cell, but exists in heteromeric complex with molecular chaperones and co-chaperones. In the functionally mature form of the heterocomplex, the free GR is associated with an Hsp90 dimer, with p23 and with any of the Hsp90 co-chaperones, except Hsp70/Hsp90 organizing protein. Such associations are essential for keeping the receptor in the correct folding for a hormone-responsive state[22]. Upon ligand binding, receptors undergo conformational changes and expose the DBD, which is otherwise hidden in the ligand-free conformation. Cytosolic receptors also provide nuclear localization signals (NLSs) that are comprised of a closely spaced arrangement of 5-8 basic amino acids[23], which co-localize with DBD and, once exposed, interact with nuclear transport factors (importins).
Nuclear heterocomplex translocation is necessary for transactivating target genes. Translocation is a tightly regulated process that occurs through nuclear pore complexes in a ligand- and energy-dependent manner, and is mediated by specific nuclear transport factors that belong to the evolutionarily conserved importin β family of nuclear transporters[23]. Among these, importin-13 (IPO13) has been functionally characterized as a primary regulator of the translocation of the GC-bound GR across the nuclear membrane[23].
The zinc finger motifs of the DBD allow the interaction of the activated receptor with specific DNA sequences, termed GC-responsive elements (GRE, 5’-GGTACAnnnTGTTCT-3’ where n refers to any nucleotide[24]), located within regulatory regions of GC-responsive genes. GR homodimerizes on GRE and recruits transcriptional co-activators, as well as basal transcription machinery, to the transcription start site. These co-activators, that include CREB (cAMP response element-binding) binding protein (CBP), steroid receptor co-activator-1 (SRC-1), GR-interacting protein (GRP-1), p300 and switching/sucrose non fermenting chromatin remodeling complex (SWI/SNF)[25], induce histone acetylation, thus allowing the transactivation of GC-responsive genes. Although some GC anti-inflammatory effects are achieved through induction of anti-inflammatory genes, such as interleukin (IL)-10, annexin 1 and the inhibitor of nuclear factor (NF)-κB[26,27], transactivation enhances mainly the expression of genes involved in metabolic processes[28,29], and is therefore, responsible for the majority of unwanted side effects[30,31].
Although positive GREs mediate transcriptional upregulation in response to GCs, negative GREs (5’-ATYACnnTnTGATCn-3’[32]) downregulate the transcription of responsive genes. Indeed, the presence of GR on GRE might competitively prevent the binding of activator protein (AP)-1 and NF-κB on the same promoter regions or might transactivate their inhibitor proteins. Furthermore, GRE-independent mechanisms of transrepression also exist: the GR physically interacts with AP-1[33], NF-κB[34] and signal transducers and activators of transcription[35]. Transrepression is believed to be responsible for the majority of the beneficial anti-inflammatory effects of GCs[30,36-38].
The molecular mechanisms of GC action are further complicated by the realization that these hormones can also induce rapid non-genomic effects within the cytoplasm; for example, they induce the release of Src kinase from the GR heterocomplex, which results in lipocortin activation and inhibition of arachidonic acid release[39], and altered cytoplasmic ion content[40].
The phenomenon of GC resistance in chronic inflammatory diseases is, as stated above, quite common, however the precise molecular mechanism is still unclear. First of all, it should be separated from the rare familial condition of primary generalized GC resistance, for which the name of Chrousos syndrome has been recently proposed[41]. This is a rare, sporadic or familiar syndrome caused by mutations in the nuclear receptor subfamily 3, group C, member 1 (NR3C1, Nuclear Receptor Nomenclature Committee, 1999) gene. The disease is characterized by target tissue insensitivity to GCs due to reduction or lack of functional GC receptors and by compensatory elevation in adrenocorticotropic hormone (ACTH). This results in an increased secretion of cortisol, albeit in the absence of signs of Cushing syndrome, as well as of other adrenal hormones with mineralocorticoid and androgenic activities, which is responsible for the main symptoms (hypertension and signs of hyperandrogenism). As stated above, this syndrome is, however, extremely rare, and no cases in IBD patients have been described in the literature.
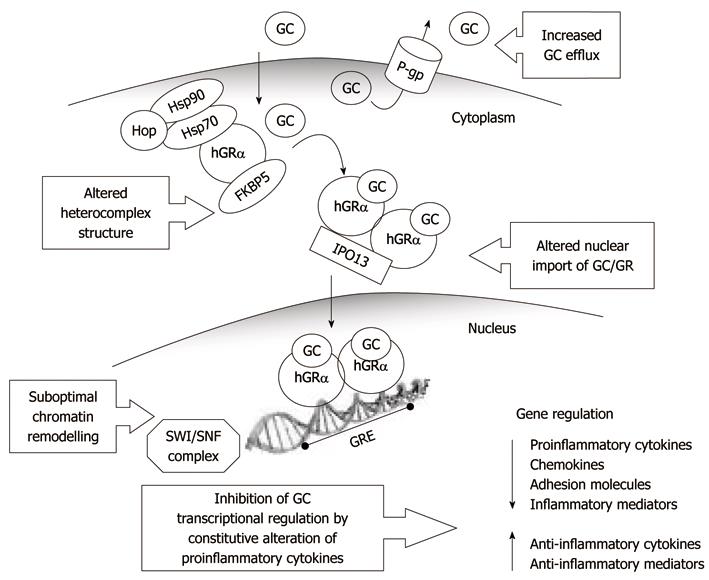
In consideration of the complexity of GC mechanisms of action (Figure 1), the most common forms of resistance observed in chronic inflammatory conditions, and in IBD in particular, may occur at several levels, and some candidate areas have been identified (Tables 1 and 2): (1) the GR receptor heterocomplex and proteins involved in nuclear translocation and transcription; (2) the pro-inflammatory mediators in the downstream signaling pathway of the GC-GR complex; and (3) P-glycoprotein (P-gp) and other proteins involved in the extrusion and metabolism of GCs.
| GR | Reduced GR mRNA expression[42] |
| Reduced GC binding affinity[43] | |
| Polymorphisms in the NR3C1 gene[45] | |
| GR heterocomplex | Altered expression of the chaperones and co-chaperones that make up the heterocomplex[74-78] |
| Polymorphisms in the Hsp90 gene[79] | |
| Genetic variations in the STIP1 gene coding for Hop[79] | |
| Nuclear transport factors | Polymorphisms in the Importin 13 gene[86] |
| Transcription machinery | Lower expression of SMARCB1 in resistant leukaemia cells[97] |
| Pro-inflammatory mediators | Polymorphisms in the NALP1 gene[72] |
| TNF-α promoter SNP correlated with steroid response in several disease[149-154] | |
| IL-10 polymorphisms related with a positive prednisone response in children with ALL[159] | |
| P-gp | Hyperexpression of P-gp in lymphocytes and epithelial cells from GC-resistant IBD patients[167] |
| GR | Significantly lower expression of GR mRNA in the intestinal mucosa in patients with GC-resistant IBD[42] |
| In colonic biopsies of UC patients, significantly more GRβ positive cells in the resistant group than in the GC sensitive control group[60] | |
| Significantly higher frequency of BclI polymorphism in GC-responsive IBD patients[61] | |
| Pro-inflammatory mediators | TNF-α G-308A polymorphism significantly associated with steroid resistance and requirement for surgery[152] |
| Association between this polymorphism and steroid dependency in a large population of CD patients[155] | |
| Higher probability of non-response to GCs in pediatric IBD patients carriers of the NALP1 variant genotype[72] | |
| P-gp | Hyperexpression of P-gp in circulating lymphocytes and epithelial cells from patients with GC resistant IBD[167] |
| Association between GC refractory CD and P-gp intronic polymorphisms[173] | |
| C3435T polymorphism in pediatric IBD Italian patients treated with GC not associated with response to therapy[152] |
The GR is an interesting candidate for GC resistance in IBD, and a significant lower expression of GR mRNA has been reported in the intestinal mucosa in patients with steroid-resistant UC[42]; in addition, GC binding affinity in mononuclear cells is reduced in patients with GC resistant diseases[43].
GR: The human GR gene NR3C1 is located on chromosome 5q31.3 and includes nine exons[44]. Polymorphisms, that is, variations in the DNA sequence with a frequency of more than 1% in the healthy population, of the human GR gene may impair the formation of the GC-GR complex and subsequently alter transactivation and/or transrepression processes.
A large number of polymorphisms in this gene have been described: according to the dbSNP build 130 database of National Center for Biotechnology Information (NCBI), 1152 entries are known at the moment, but only a few are functionally relevant. The TthIIII (rs10052957), ER22/23EK (rs6189/rs6190), N363S (rs6195), BclI (rs41423247) and GR-9β (rs6198) polymorphisms have been the most studied and have been associated with differences in metabolic parameters and body composition, and with autoimmune and cardiovascular disease. These genetic variants have been also related with changes in GC sensitivity or altered cortisol level[45] and may therefore account for the variability in the response to GC therapy.
Three polymorphisms are associated with a reduced sensitivity to endogenous and exogenous GCs.
TthIIII is a restriction fragment length polymorphism (RFLP) caused by a C>T change in the GR gene promoter region; it is located in a large intron of approximately 27 kb, 3807 bp upstream of the GR start site[22]. The polymorphism has been associated with elevated diurnal cortisol levels[46] and with a reduced cortisol response to 1 mg dexamethasone (DEX), as well as lower insulin and cholesterol levels[47].
The ER22/23EK polymorphisms are located in the N-terminal transactivation domain of the GR and involve two nucleotide changes in codon 22 and 23 of exon 2 (GAG AGG to GAA AAG), which are changing the amino acid sequence from glutamic acid-arginine (E-R) to glutamic acid-lysine (E-K). Since the polymorphism is located in the transactivation domain, the amino acid change might affect the tertiary structure of the receptor, which influences the transactivational and/or transrepressional activity on target genes[48]. A relative GC resistance, with a reduction of GR transcriptional activity in transfected COS-1 cells and in peripheral blood mononuclear leukocytes of homozygous carriers, has been described[49]. In vivo, an association with higher post-DEX cortisol levels as well as less cortisol suppression after a 1 mg DEX suppression test in ER22/23EK carriers, has also been shown. In addition, the polymorphism is associated with a better metabolic and cardiovascular health profile and an increased survival[50,51].
The GR-9β polymorphism is located in the 3’-untranslated region (UTR) of exon 9β, where an ATTTA sequence is changed into GTTTA. This polymorphism was first characterized by Derijk et al[52] and functional studies have revealed a stabilizing effect on the mRNA of the GRβ isoform, which leads to enhanced expression of the inactive GRβ protein. GRβ is one of several GR protein isoforms and is generated through an alternative splicing pathway that links further downstream sequences of exon 9, termed exon 9β, to the end of exon 8. In contrast to the functionally active and most abundant isoform GRα[53], GRβ is unable to bind ligand, is transcriptionally inactive, and exerts a dominant negative effect on transactivation by interfering with the binding of GRα to the DNA[54-58]. Honda et al[59] have reported GRβ-specific mRNA expression in lymphocytes of 83% of patients with steroid-resistant UC, compared to only 9% in responsive subjects and 10% in healthy controls and chronic active CD patients. This observation has recently been confirmed by Fujishima et al[60] who have found, in colonic biopsies of UC patients, significantly more GRβ-positive cells in the resistant group than in the GC sensitive and control groups. However, in IBD, GRβ is expressed 100-1000 times less than GRα, and this challenges its role in the genesis of steroid resistance in this disease.
Only a few studies, so far, have evaluated the role of the TthIIII, ER22/23EK and GR-9β polymorphisms in the response to exogenous GCs in IBD or in other diseases. In 119 pediatric patients with IBD, no association has been found between the ER22/23EK polymorphism and GC response[61]. In addition, the polymorphism did not appear to confer protection against the occurrence of respiratory distress syndrome in 62 preterm infants born to mothers treated with a complete course of betamethasone[62], or to play a role in the toxicity induced by GCs in 36 children with acute lymphoblastic leukemia (ALL)[63]. The three polymorphisms have been also studied in haplotype: in 646 patients with multiple sclerosis treated with GCs, the haplotype consisting in TthIIII, ER22/23EK, and 9β-G was associated with GC resistance, with a more rapid disease progression. However this seemed to result from the presence of ER22/23EK, and not from the other two polymorphisms[64].
Two single nucleotide polymorphisms (SNPs) in the NR3C1 gene, the N363S and BclI polymorphisms, are associated with an increased sensitivity to GCs.
The N363S polymorphism is located in exon 2 and consists of an AAT>AGT nucleotide change at position 1220, which results in an asparagine to serine change in codon 363. A significantly higher transactivating capacity of the mutant has been described in vitro in human peripheral blood mononuclear cells[65] and is associated with an increased sensitivity to GCs in vivo[66]. Few studies have investigated the role of this polymorphism in the response to exogenous GCs. Szabó et al[67] have investigated whether variants of the NR3C1 gene may contribute to steroid-induced ocular hypertension. In 102 patients who underwent photorefractive keratectomy and received topical steroids as part of postoperative therapy, a significant correlation was found between N363S heterozygosity and ocular hypertension. Furthermore, in 48 patients with Duchenne muscular dystrophy treated with prednisolone or deflazacort, the N363S carriers showed a trend towards a later age at loss of ambulation in comparison with non-carrier patients[68]. Only one study so far has evaluated the role of this polymorphism in GC response in IBD: but no relation was observed between the presence of this SNP and response to GCs in a population of 119 pediatric patients[61].
As extensively reported in the literature, the most clinically relevant polymorphism of the NR3C1 gene is the BclI SNP. Initially described as a polymorphic restriction site inside intron 2, the nucleotide alteration was subsequently identified as a C>G substitution, 646 nucleotides downstream from exon 2[69]. This polymorphism is also associated with hypersensitivity to GCs in both heterozygous and homozygous carriers of the G allele. An association with unfavorable metabolic characteristics, such as increased body mass index and insulin resistance has been also described[70,71].
The BclI SNP has been studied in 119 pediatric patients with IBD (64 with CD, and 55 with UC). Patients were divided into two groups based on their response to GC treatment: GC dependence (45 patients) was defined by an initial response to prednisone with relapse on dose reduction, not allowing steroid discontinuation, and GC-responsiveness (67 patients), equivalent to therapeutic success, was defined as GC withdrawal without the need for steroids for at least 1 year. A significantly higher frequency of BclI mutated genotype was observed in the GC-responsive patients than in the GC-dependent group, which confirms an increased sensitivity to GCs in subjects with the BclI mutated genotype[61]. These results have been subsequently confirmed in a larger cohort of young patients with IBD[72].
GR heterocomplex: The integrity of the mature GR heterocomplex is required for optimal ligand binding and subsequent activation of the transcriptional response, and abnormalities in the chaperones and co-chaperones that make up the heterocomplex may contribute to altered GC responsiveness[73].
Although no study has considered the role of these abnormalities in IBD, altered levels of Hsp90 have been found in peripheral blood mononuclear cells from individuals with steroid-resistant forms of asthma[74], multiple sclerosis[75], and idiopathic nephrotic syndrome[76]. Kojika et al[77] have shown that alteration in Hsp90 and Hsp70 is associated with decreased sensitivity to GCs in human leukemia cells. However, no relationship has been found between Hsp90 mRNA and resistance to GCs in spare bone marrow or peripheral blood samples taken from ALL patients at diagnosis[78]. SNPs in the Hsp90 genes (HSPCA encoding hsp90-1α, HSPCB encoding hsp90-1β) have been recently described; however, in an adult asthmatic population, no correlation was found between SNPs in the HSPCA (3’-UTR+307, rs3736807, rs4906178, rs3809386, promoter -32) or HSPCB (rs504697 and rs3757286) genes and response to treatment[79].
Generally, steroid receptors display functional instability when deprived of chaperones. FKBP51, coded by the FKBP5 gene, is a negative regulator of GC action and reduces GR binding affinity[80]. However, when GCs binds to the GR, the receptor complex is activated and FKBP51 is replaced by FKBP52 (coded by FKBP4 gene), a positive regulator of GR signaling[81,82]. The relative levels of FKBP51 and FKBP52 have been shown to be important determinants of GC cellular sensitivity in various systems. One recent study has investigated the role of FKBP5 genetic variants in the response to GCs in asthmatic patients[79], however the studied polymorphisms (rs3800373, rs9394309, rs938525, rs9470080, rs9368878 and rs3798346) were not correlated with response. In this study, genetic variations in the STIP1 gene, which codes for the co-chaperone Hsp70/Hsp90 organizing protein, on the contrary, seemed to have a role in identifying asthmatic subjects who were more responsive to GC therapy[79].
Nuclear transport factors: Importins represent important players in GC pharmacology, as they mediate nuclear translocation through nuclear pore complexes. Importin 13 (IPO13) has been functionally characterized as a primary regulator of GC-bound GR across the nuclear membrane[23]. IPO13 was first discovered as a GC-inducible gene that is important in lung development[83], therefore, its functional characterization has been performed in airway epithelial cells and lung-derived transformed cell lines[84]. Inhibition of lung epithelial cell IPO13 production reduces the nuclear translocation of GR from the cytoplasm, and subsequent GC-mediated silencing of inflammatory cytokine production[84], which suggests that the normal anti-inflammatory response induced by GCs is dependent on normal IPO13 expression levels or activity. Therefore, irregular GC-GR signaling, ascribable to IPO13 variation, might affect the therapeutic responsiveness to GCs. IPO13 is expressed in other tissues as well, such as fetal brain, heart, kidneys and intestine[83,85], therefore, it is feasible to hypothesize that dysregulation of IPO13-mediated processes might account for the variable response to GCs also in other diseases, such as IBD.
Importins are interesting candidates for pharmacogenomic studies, although little is known about their genetic variants. Recently, the association of 10 IPO13 polymorphisms with responsiveness to inhaled GCs, measured by change in methacoline dose required for a 20% drop in FEV1, has been investigated in children with mild to moderate asthma[86]. Unexpectedly, the genetic effects conferred by IPO13 variants were clinically significant in subjects who were randomized to placebo and nedocromil treatment (control group), rather than those treated with the inhaled GC budenoside, either in single SNP or in haplotype analysis. No other study on IPO13 genetic variants is available to date, therefore, these results require confirmation and further investigations are needed to shed light on the putative role of IPO13 mutants in GC-resistant or GC-hyper-responsive cases in other chronic inflammatory diseases.
Transcription machinery: In eukaryotic cells, gene transcription is inhibited by condensed chromatin structure, which prevents the interaction between gene-specific transcription factors and their DNA recognition sequences, thus blocking the access of the transcriptional machinery to DNA[87]. Dynamic chromatin remodeling is therefore a fundamental mechanism in mediating genomic effects, and a large family of protein complexes that promote chromatin restructuring in an ATP-dependent manner, by disrupting histone-DNA contacts, has been described[88-90]. Among these, the highly conserved mammalian SWI/SNF chromatin remodeling complex is the one recruited during GC-dependent gene activation[91-93]. The complex is composed of several subunits[94,95], including a catalytic ATPase subunit (either SMARCA4/BRG1 or SMARCA2/BRM) and Brahma-related gene 1 (BRG1)-associated proteins[96], such as the SWI/SNF-related, matrix-associated, actin-dependent regulators of chromatin (SMARC)C1, SMARCC2, SMARCD1-3, SMARCE1, SMARCB1 and ACTL6-A-B[94,95].
Alterations in any component of the SWI/SNF complex might be responsible for GC resistance, if access of GC-bound GR to DNA is compromised because of an impaired or suboptimal chromatin remodeling and nucleosome disruption. Using a genome-wide approach in patients with newly diagnosed childhood B-lineage ALL, differential expression of a relatively small number of genes has been shown to be associated with drug resistance and treatment outcome[97]. Lower expression of SMARCB1 is related to resistant leukemia cells and, vice versa, higher levels are associated with GC-sensitive ALL[97]. Decreased expression of other core subunits of the SWI/SNF complex, such as SMARCA4 and ARID1A, is also associated with GC resistance in primary ALL cells[98].
To date, only a few studies have addressed the functional effects of SMARC polymorphisms. By using the human CEPH (Centre d’Etude du Polymorphisme Humain) cell lines from 90 individuals and sequencing the SMARCB1 promoter region, a regulatory SNP (-228G>T) has been discovered: the TT genotype has been functionally associated with higher SMARCB1 expression at both mRNA and protein levels[99], and a positive association has been found between the SMARCB1 mRNA level and prednisolone sensitivity, as evaluated by MTT assay. In addition, knockdown of SMARCB1 in human ALL cell lines has confirmed that reduced SMARCB1 expression induces GC resistance[99].
Inflammation can be seen as a physiological homeostatic process elicited by the body in response to injurious agents, to protect tissues and to help in recovery. However, inflammation itself can potentially lead to tissue damage, if the organism does not properly control the process. Endogenous GCs are involved in the regulation of many physiological functions and assist the innate and adaptive immunity[100-102] by balancing pro- and anti-inflammatory mediators, thus preventing overwhelming inflammation[103].
According to gene expression analysis of human peripheral blood mononuclear cells from healthy donors, approximately 20% of genes, particularly those involved in the immune response, are regulated either positively or negatively in response to DEX treatment[104]. GCs downregulate the expression of pro-inflammatory cytokines [such as IL-1α, IL-1β, IL-8, interferon (IFN)-α and IFN-β[104,105]], chemokines[106,107], adhesion molecules, inflammatory enzymes and receptors. At the same time, GCs upregulate the expression of other cytokines that suppress the production of inflammatory mediators (such as transforming growth factor-β3 and IL-10), thus boosting the anti-inflammatory effects.
Pro-inflammatory cytokines are involved in the pathogenesis of several chronic inflammatory diseases such as rheumatoid arthritis, osteoarthritis, asthma and IBD[108,109]. Their excessive expression is generally efficiently counteracted by GCs[110]. Individuals with steroid-non-responsive forms often show higher levels of local and/or systemic pro-inflammatory cytokines[111-113].
Research of the cytokine profile in relation to GC resistance has been mainly performed in steroid-resistant asthma, and only a few studies have involved patients with IBD. Several in vitro data suggest that cytokines modify GC effects by interference with the GR signaling[114]. IL-1α has been reported to inhibit DEX-induced GR translocation in mouse fibroblasts[115] and to downregulate the GR in a rat hepatoma cell line[116], whereas IL-1β inhibits GR function in colonic epithelial cells[117]. Tumor necrosis factor (TNF)-α decreases corticosensitivity in monocytes by downregulation of GR[118], and, in patients with UC, mucosal levels of this cytokine, as well as of IL-6 and IL-8 are higher in steroid-resistant patients[119]. In a recent study, a large panel of cytokines was studied and their expression was correlated with steroid response. In particular, high IL-10 expression significantly enhanced steroid action, while IL-2 appeared to have the greatest antagonistic effect on the antiproliferative activity of steroids[19]. This cytokine has been also related to increased GRβ expression[120], decreased translocation of the GR-GC complex to the nucleus[121], and increased AP-1 levels, with abnormal interaction with the GR[122]. IL-2 and IL-4 reduce GR affinity and T-cell response to GCs in vitro[43], by a mechanism that involves p38 mitogen activated protein kinase (MAPK) activation[123]. GCs inhibit the MAPK signaling pathway, through the induction of MAPK phosphatase 1 (MKP1), and this could result in inhibition of expression of various inflammatory genes[124]. In conclusion, a complex circular interplay between GCs and cytokines takes place, with GCs downregulating pro-inflammatory cytokines and cytokines limiting GC action.
Basal cytokine expression levels are fine-tuned by genetic profile. Polymorphisms in the cytokine regulatory regions determine a “lower/higher cytokine producers” phenotype that might in part be responsible of inter-individual variations, in terms of severity of inflammation and therapy responses. Higher cytokine producers might become GC-resistant or they might be less responsive at standard doses, therefore requiring dose adjustment to overcome the inflammation; however, only a few studies have focused on the effect of these genetic polymorphisms on GC response.
IL-1β: Among the major inflammatory cytokines, IL-1 has a pivotal role. IL-1 is constituted by two distinct polypeptides, IL-1α and IL-1β. IL-1β can promote the production of matrix metalloproteinases and the synthesis of prostaglandins[110,125], as well as the production of other inflammatory mediators such as IL-6, IL-8 and TNF-α, thus amplifying the inflammation cascade[126]. IL-1β is therefore a key player in prompting and maintaining inflammation[119,127] and polymorphisms in the IL-1β gene might be of primary relevance in the modulation of GC response.
A cluster of genes encoding for IL-1α, IL-1β and for the natural receptor antagonist IL-1Ra has been mapped on chromosome 2q13[128,129]. Polymorphisms in this gene cluster have been associated with a large variety of human diseases[130] and with altered levels of IL-1[131-134]. So far, the dbSNP build 130 database of NCBI reports 158 submissions for human IL-1, among which, several SNPs are described[131,135]. Two SNPs in the IL-1β promoter region (C-511T and T-31C) exhibit almost complete linkage disequilibrium. The C-511T SNP (rs16944) results in the loss of AP-2 binding site, while the T-31C (rs1143627) results in the loss of the first T in TATA box. The latter SNP appears to cause a paradoxical increase in IL-1β in the presence of steroids[136], which could be relevant to the occurrence of GC resistance. Carriers of the haplotype composed of IL-1β -31C allele and -511T allele showed higher plasmatic concentrations of the cytokine, compared to subjects with IL-1β wild-type genotype in Caucasians[132,137], but decreased mRNA and protein levels in Japanese subjects[138]. A recent report from this laboratory has investigated the role of the C-511T polymorphism in the response to GCs in 154 young IBD patients, but no relation with GC resistance (14 patients) or dependence (54 patients) was found[72].
IL-1β is released as an inactive precursor (pro IL-1β, p35) by monocytes and macrophages in response to inflammatory stimuli, and is cleaved to the active mature form (p17) by caspase-1[139,140]. Caspase-1 is part of multiprotein cytoplasmic complexes, called NACHT leucine-rich-repeat protein 1 (NALP1) and NALP3 inflammasomes. Jin et al[141,142] have recently shown that variants of NALP1 are associated with several autoimmune diseases, and have suggested that mutations in NALP1 gene may result in deregulated secretion of IL-1β. The rs12150220 non-synonymous polymorphism, previously reported to confer susceptibility to autoimmune and autoinflammatory diseases[143] results in the Leu > His amino acidic variation in position 155, between the N-terminal pyrin and NACHT domains of the human NALP1 protein. This region has been highly conserved through primate evolution, which suggests a critical role in protein function[142]. Data from our laboratory have shown that pediatric IBD patients, carriers of the NALP1 homozygote rs12150220 variant genotype, exhibit a higher probability of non-response to GC therapy[72].
TNF-α: TNF-α is a potent pro-inflammatory cytokine released by cells of the immune system upon stimulation, and is almost not detectable in resting conditions[144]. The gene encoding TNF-α is located in the class III region of the major histocompatibility complex within the human leukocyte antigen (HLA) on chromosome 6p21.3[145]. This part of the genome is one of the most polymorphic in humans and contains many genes that encode proteins involved in inflammatory and immune responses[146].
The G-308A (rs1800629) is one of the best documented polymorphisms of the TNF-α gene[147]. This SNP lies in a binding site for the transcription factor AP-1 and the A allele has been shown to have higher transcriptional activity than the G allele, increasing TNF-α production in vitro[148]. The A allele carriers show an enhanced susceptibility to several autoimmune and inflammatory disorders, such as systemic lupus erythematosus, celiac disease[149], Alzheimer disease[150,151], IBD[152], asthma[153] and rheumatoid arthritis[154].
The polymorphism has been correlated also with steroid response in several diseases. In a cohort of 386 pediatric IBD patients, the mutated allele was significantly associated with steroid resistance and requirement for surgery in the subset of 200 CD patients[152]. In addition, previous evidence in a large population of CD patients has shown that this polymorphism is more frequent in steroid-dependent subjects; possibly as a consequence of a more intense TNF-α-driven inflammatory reaction at the mucosal level[155]. The AA genotype has been associated with reduced response to steroids also in other diseases, such as idiopathic nephrotic syndrome[156], rejection episodes in HLA-DR mismatched transplant patients[157] and solid and lymphoid malignancies[158,159].
IL-10: IL-10, known as human cytokine synthesis inhibitory factor, is a cytokine that is produced primarily by monocytes and to a lesser extent by lymphocytes. IL-10 has pleiotropic effects in immunoregulation and inflammation[160-162]. It inhibits the production of inflammatory mediators, and can be considered as a natural immunosuppressant of TNF[163]. GCs upregulate the expression of IL-10[104], and conversely, IL-10 acts synergistically with GCs, improving the ability of DEX to reduce IL-6 secretion in whole-blood cell cultures. In addition, the cytokine increases the concentration of DEX-binding sites in these cells, with no effect on the binding affinity[118]. The human IL-10 gene is located on chromosome 1q31-q32. Previous studies have demonstrated that an A>G polymorphism at nucleotide position -1082 within the IL-10 gene promoter region (rs1800896) influences the cytokine plasma levels, which are significantly higher in patients homozygous for the G allele[159]. No data exist on the role of IL-10 polymorphisms in GC response in IBD, however, the mutated genotype is related to a positive prednisone response in childhood ALL[159]. In accordance, Marino and colleagues[63] have found a better response to GC remission induction therapy in Italian pediatric ALL patients with the mutated allele. In patients affected by rheumatoid arthritis, the high IL-10 producer genotype (-1082GG) is also associated with a favorable outcome, specifically to prednisone therapy[164].
Cellular extruding pumps and metabolizing enzymes are the two key pathways involved in the elimination of many drugs. P-gp is one of the major transporters that extrude xenobiotics and drugs out of the cells. The cytochrome P450 (CYP) enzyme superfamily, that catalyzes phase I
reactions, is active in the metabolism of many exogenous and endogenous compounds. P-gp and CYP3A are expressed in the intestinal epithelial cells, in lymphocytes and in the liver and have been reported to be involved in the transport and metabolism of GCs.
P-gp: P-gp is a 170-kDa transport membrane glycoprotein, which is responsible for resistance to a number of structurally and functionally unrelated drugs in clinical use. Human P-gp is a phosphorylated and glycosylated protein that consists of 1280 amino acids and two homologous and symmetric sequences, each containing six transmembrane domains and an ATP-binding motif. ATP hydrolysis provides the energy for active drug transport against a steep concentration gradient[165]. The protein plays an important role in the absorption, distribution, and elimination of drugs that are its substrates, among which GCs, and P-gp inhibitors reduce cortisol efflux from human intestinal epithelial cells and T cells[166]. The protein is highly expressed in circulating lymphocytes and epithelial cells from CD and UC patients with GC-resistant IBD[167], however a relationship between GC administration and P-gp expression has been described in monocytes from IBD patients[168]. It remains therefore to be clarified if the increased expression of P-gp reflects a primary phenomenon in IBD or a secondary one, influenced by GC therapy.
P-gp is encoded by the ABCB1 (MDR1) gene, located on human chromosome 7q21.12[169], and several studies have demonstrated that genetic polymorphisms in this gene lead to functional alterations and phenotypic variations in P-gp expression[170,171]. The first systematic screening for ABCB1 polymorphisms was performed in 2000[170]: a synonymous SNP in exon 26 (C3435T) was the first variation to be associated with altered protein expression. P-gp expression in the duodenum of individuals with the homozygous variant T allele was decreased when compared with individuals with the C allele (wild-type). Other studies have shown that SNPs at exons 12 (C1236T), 21 (G2677T/A) and 1b (T-129C) may also be associated with altered transport function or expression[172]. A weak association between GC refractory CD and MDR1 polymorphisms in introns 13 and 16 has been initially described[173], however, subsequent studies have not confirmed this observation. In 2007, Cucchiara and colleagues studied the C3435T MDR1 polymorphism in 200 pediatric Italian patients with CD and 186 patients with UC treated with GCs, and demonstrated that this SNP was not associated with response to medical therapy[152]. In confirmation of these results, a large study performed on an adult Italian IBD population did not find any association between the polymorphisms of the MDR1 gene (C3435T and G26677T/A) and clinical response to GC therapy[174]. Further studies are therefore needed to clarify if MDR1 polymorphisms play a role in steroid resistance in IBD.
CYP3A: CYP3A is the primary CYP subfamily in humans and is responsible for the phase I metabolism of > 50% of drugs currently in use[175], including GCs[176]. Many drug substrates of CYP3A4 are also substrates of P-gp, and the overlap between CYP3A4 and P-gp is also emphasized by the genomic proximity of these genes (7q22.1 and 7q21.1 for CYP3A4 and MDR1, respectively)[172]. The CYP3A activity of the adult human liver is the sum activity of all subfamily members, including CYP3A5, which is highly polymorphic in Caucasians[177], and genetic variants may play an important role in inter-individual differences in the pharmacokinetics of drugs metabolized by CYP3A5.
The most frequent and functionally important SNP in the CYP3A5 gene consists of an A6986G transition within intron 3 (CYP3A5*3)[175]: this mutation creates an alternative splice site in the pre-mRNA and results in production of an aberrant mRNA (SV1-mRNA) that contains 131 bp of intron 3 sequence (exon 3B) inserted between exons 3 and 4. The exon 3B insertion turns out in a frameshift and predicted truncation of the translated protein at amino acid 102. Additional intronic or exonic mutations (CYP3A5*5, *6, and *7) may alter splicing and result in premature stop codons or exon deletion[178,179].
No report is present in the literature about the role of CYP3A5 polymorphisms in GC resistance in IBD, but plasma prednisolone concentrations, measured by HPLC in 95 renal transplant recipients treated with repeated doses of triple therapy immunosuppression, consisting of prednisolone, tacrolimus and mycophenolate mofetil, have been recently studied[180]. The AUC0-24 of prednisolone in recipients having both MDR1 3435CC and CYP3A5*3/*3 genotypes tends to be higher than the MDR1 3435TT plus CYP3A5*3/*3 genotype. The CYP3A5*3 polymorphism has also been included in a study to identify pharmacogenomic predictors of outcome in 70 pediatric heart transplant patients followed for at least 1 year post-transplantation, but in this population, no correlation of the CYP3A5*3 SNP with steroid response after transplantation was observed[181].
GCs are potent anti-inflammatory drugs and, despite considerable adverse effects, still remain the first-line therapy for inducing a rapid remission in moderate to severe active CD and UC. In addition, high inter-individual variability is observed when these agents are administered to patients with IBD and other chronic inflammatory diseases. A main goal in this area of medicine is therefore to improve the efficacy and safety of these agents and, when possible, to reduce steroid exposure and to employ a non-steroid option. This is particularly important in patients that do not respond, who will suffer considerable steroid-dependent morbidity without any clinical gain.
Pharmacogenomics is a relatively novel branch of investigation and represents an innovative frontier of medicine. Its ambitious goal is to identify genetic determinants that will help physicians in diagnostic decisions, and supply reliable genetic tools to adjust treatment a priori. A knowledge of genetic profiles with an impact on drug response would improve cure rates, avoid inadequate regimens or time wasting, and reduce overall health-care costs.
Pharmacogenetic research in IBD has witnessed only modest success; in particular, because treatment response in this disease is influenced by many factors, such as disease duration, behavior and severity, and at present, despite intensive investigation, none of the potential pharmacogenetic markers is strong enough to be used in clinical practice. Reported genetic associations have not yet shown consistent or robust results, and for most of the considered SNPs, results are controversial. More studies with larger sample size and well-characterized patient cohorts, uniformly treated and systematically evaluated, are therefore needed. These studies should support findings with greater statistical confidence that should be hopefully translated into clinical practice in the near future.
Peer reviewer: Masahiro Iizuka, MD, PhD, Director, Akita Health Care Center, Akita Red Cross Hospital, 3-4-23, Nakadori, Akita, 010-0001, Japan
S- Editor Tian L L- Editor Kerr C E- Editor Zheng XM
| 1. | Kaspers GJ, Pieters R, Van Zantwijk CH, Van Wering ER, Van Der Does-Van Den Berg A, Veerman AJ. Prednisolone resistance in childhood acute lymphoblastic leukemia: vitro-vivo correlations and cross-resistance to other drugs. Blood. 1998;92:259-266. [Cited in This Article: ] |
| 2. | Boumpas DT, Chrousos GP, Wilder RL, Cupps TR, Balow JE. Glucocorticoid therapy for immune-mediated diseases: basic and clinical correlates. Ann Intern Med. 1993;119:1198-1208. [Cited in This Article: ] |
| 3. | Barnes PJ, Adcock I. Anti-inflammatory actions of steroids: molecular mechanisms. Trends Pharmacol Sci. 1993;14:436-441. [Cited in This Article: ] |
| 4. | de Vries F, Pouwels S, Lammers JW, Leufkens HG, Bracke M, Cooper C, van Staa TP. Use of inhaled and oral glucocorticoids, severity of inflammatory disease and risk of hip/femur fracture: a population-based case-control study. J Intern Med. 2007;261:170-177. [Cited in This Article: ] |
| 5. | Souverein PC, Berard A, Van Staa TP, Cooper C, Egberts AC, Leufkens HG, Walker BR. Use of oral glucocorticoids and risk of cardiovascular and cerebrovascular disease in a population based case-control study. Heart. 2004;90:859-865. [Cited in This Article: ] |
| 6. | Vegiopoulos A, Herzig S. Glucocorticoids, metabolism and metabolic diseases. Mol Cell Endocrinol. 2007;275:43-61. [Cited in This Article: ] |
| 7. | Chung KF, Godard P, Adelroth E, Ayres J, Barnes N, Barnes P, Bel E, Burney P, Chanez P, Connett G. Difficult/therapy-resistant asthma: the need for an integrated approach to define clinical phenotypes, evaluate risk factors, understand pathophysiology and find novel therapies. ERS Task Force on Difficult/Therapy-Resistant Asthma. European Respiratory Society. Eur Respir J. 1999;13:1198-1208. [Cited in This Article: ] |
| 8. | Barnes PJ, Woolcock AJ. Difficult asthma. Eur Respir J. 1998;12:1209-1218. [Cited in This Article: ] |
| 9. | Markowitz J, Grancher K, Kohn N, Lesser M, Daum F. A multicenter trial of 6-mercaptopurine and prednisone in children with newly diagnosed Crohn's disease. Gastroenterology. 2000;119:895-902. [Cited in This Article: ] |
| 10. | Hyams J, Markowitz J, Lerer T, Griffiths A, Mack D, Bousvaros A, Otley A, Evans J, Pfefferkorn M, Rosh J. The natural history of corticosteroid therapy for ulcerative colitis in children. Clin Gastroenterol Hepatol. 2006;4:1118-1123. [Cited in This Article: ] |
| 11. | Munkholm P, Langholz E, Davidsen M, Binder V. Frequency of glucocorticoid resistance and dependency in Crohn's disease. Gut. 1994;35:360-362. [Cited in This Article: ] |
| 12. | Faubion WA Jr, Loftus EV Jr, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255-260. [Cited in This Article: ] |
| 13. | Hearing SD, Norman M, Probert CS, Haslam N, Dayan CM. Predicting therapeutic outcome in severe ulcerative colitis by measuring in vitro steroid sensitivity of proliferating peripheral blood lymphocytes. Gut. 1999;45:382-388. [Cited in This Article: ] |
| 14. | Corrigan CJ, Brown PH, Barnes NC, Szefler SJ, Tsai JJ, Frew AJ, Kay AB. Glucocorticoid resistance in chronic asthma. Glucocorticoid pharmacokinetics, glucocorticoid receptor characteristics, and inhibition of peripheral blood T cell proliferation by glucocorticoids in vitro. Am Rev Respir Dis. 1991;144:1016-1025. [Cited in This Article: ] |
| 15. | Seki M, Ushiyama C, Seta N, Abe K, Fukazawa T, Asakawa J, Takasaki Y, Hashimoto H. Apoptosis of lymphocytes induced by glucocorticoids and relationship to therapeutic efficacy in patients with systemic lupus erythematosus. Arthritis Rheum. 1998;41:823-830. [Cited in This Article: ] |
| 16. | Kirkham BW, Corkill MM, Davison SC, Panayi GS. Response to glucocorticoid treatment in rheumatoid arthritis: in vitro cell mediated immune assay predicts in vivo responses. J Rheumatol. 1991;18:821-825. [Cited in This Article: ] |
| 17. | Langhoff E, Ladefoged J, Jakobsen BK, Platz P, Ryder LP, Svejgaard A, Thaysen JH. Recipient lymphocyte sensitivity to methylprednisolone affects cadaver kidney graft survival. Lancet. 1986;1:1296-1297. [Cited in This Article: ] |
| 18. | Hearing SD, Norman M, Smyth C, Foy C, Dayan CM. Wide variation in lymphocyte steroid sensitivity among healthy human volunteers. J Clin Endocrinol Metab. 1999;84:4149-4154. [Cited in This Article: ] |
| 19. | Creed TJ, Lee RW, Newcomb PV, di Mambro AJ, Raju M, Dayan CM. The effects of cytokines on suppression of lymphocyte proliferation by dexamethasone. J Immunol. 2009;183:164-171. [Cited in This Article: ] |
| 20. | Schimmer B, Parker K. Adrenocorticotropic hormone; adrenocortical steroids and their synthetic analogs; inhibitors of the synthesis and actions of adrenocortical hormones. Goodman & Gilman’s The pharmacological basis of therapeutics. 11th ed. New York: McGraw-Hill 2006; 901-913. [Cited in This Article: ] |
| 21. | Escriva H, Delaunay F, Laudet V. Ligand binding and nuclear receptor evolution. Bioessays. 2000;22:717-727. [Cited in This Article: ] |
| 22. | Detera-Wadleigh SD, Encio IJ, Rollins DY, Coffman D, Wiesch D. A TthIII1 polymorphism on the 5' flanking region of the glucocorticoid receptor gene (GRL). Nucleic Acids Res. 1991;19:1960. [Cited in This Article: ] |
| 23. | Pemberton LF, Paschal BM. Mechanisms of receptor-mediated nuclear import and nuclear export. Traffic. 2005;6:187-198. [Cited in This Article: ] |
| 24. | Nordeen SK, Suh BJ, Kühnel B, Hutchison CA 3rd. Structural determinants of a glucocorticoid receptor recognition element. Mol Endocrinol. 1990;4:1866-1873. [Cited in This Article: ] |
| 25. | Smith LK, Cidlowski JA. Glucocorticoid-induced apoptosis of healthy and malignant lymphocytes. Prog Brain Res. 2010;182:1-30. [Cited in This Article: ] |
| 26. | Catley M. Dissociated steroids. Sci World J. 2007;7:421-430. [Cited in This Article: ] |
| 27. | Ehrchen J, Steinmüller L, Barczyk K, Tenbrock K, Nacken W, Eisenacher M, Nordhues U, Sorg C, Sunderkötter C, Roth J. Glucocorticoids induce differentiation of a specifically activated, anti-inflammatory subtype of human monocytes. Blood. 2007;109:1265-1274. [Cited in This Article: ] |
| 28. | Schäcke H, Schottelius A, Döcke WD, Strehlke P, Jaroch S, Schmees N, Rehwinkel H, Hennekes H, Asadullah K. Dissociation of transactivation from transrepression by a selective glucocorticoid receptor agonist leads to separation of therapeutic effects from side effects. Proc Natl Acad Sci USA. 2004;101:227-232. [Cited in This Article: ] |
| 29. | Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23-43. [Cited in This Article: ] |
| 30. | Stahn C, Löwenberg M, Hommes DW, Buttgereit F. Molecular mechanisms of glucocorticoid action and selective glucocorticoid receptor agonists. Mol Cell Endocrinol. 2007;275:71-78. [Cited in This Article: ] |
| 31. | Löwenberg M, Stahn C, Hommes DW, Buttgereit F. Novel insights into mechanisms of glucocorticoid action and the development of new glucocorticoid receptor ligands. Steroids. 2008;73:1025-1029. [Cited in This Article: ] |
| 32. | Truss M, Beato M. Steroid hormone receptors: interaction with deoxyribonucleic acid and transcription factors. Endocr Rev. 1993;14:459-479. [Cited in This Article: ] |
| 33. | Yang-Yen HF, Chambard JC, Sun YL, Smeal T, Schmidt TJ, Drouin J, Karin M. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell. 1990;62:1205-1215. [Cited in This Article: ] |
| 34. | Ray A, Prefontaine KE. Physical association and functional antagonism between the p65 subunit of transcription factor NF-kappa B and the glucocorticoid receptor. Proc Natl Acad Sci USA. 1994;91:752-756. [Cited in This Article: ] |
| 35. | Stöcklin E, Wissler M, Gouilleux F, Groner B. Functional interactions between Stat5 and the glucocorticoid receptor. Nature. 1996;383:726-728. [Cited in This Article: ] |
| 36. | Adcock IM, Caramori G. Cross-talk between pro-inflammatory transcription factors and glucocorticoids. Immunol Cell Biol. 2001;79:376-384. [Cited in This Article: ] |
| 37. | Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066-1071. [Cited in This Article: ] |
| 38. | Miner JN, Yamamoto KR. The basic region of AP-1 specifies glucocorticoid receptor activity at a composite response element. Genes Dev. 1992;6:2491-2501. [Cited in This Article: ] |
| 39. | Croxtall JD, Choudhury Q, Flower RJ. Glucocorticoids act within minutes to inhibit recruitment of signalling factors to activated EGF receptors through a receptor-dependent, transcription-independent mechanism. Br J Pharmacol. 2000;130:289-298. [Cited in This Article: ] |
| 40. | McConkey DJ, Nicotera P, Hartzell P, Bellomo G, Wyllie AH, Orrenius S. Glucocorticoids activate a suicide process in thymocytes through an elevation of cytosolic Ca2+ concentration. Arch Biochem Biophys. 1989;269:365-370. [Cited in This Article: ] |
| 41. | Charmandari E, Kino T. Chrousos syndrome: a seminal report, a phylogenetic enigma and the clinical implications of glucocorticoid signalling changes. Eur J Clin Invest. 2010;40:932-942. [Cited in This Article: ] |
| 42. | Raddatz D, Middel P, Bockemühl M, Benöhr P, Wissmann C, Schwörer H, Ramadori G. Glucocorticoid receptor expression in inflammatory bowel disease: evidence for a mucosal down-regulation in steroid-unresponsive ulcerative colitis. Aliment Pharmacol Ther. 2004;19:47-61. [Cited in This Article: ] |
| 43. | Kam JC, Szefler SJ, Surs W, Sher ER, Leung DY. Combination IL-2 and IL-4 reduces glucocorticoid receptor-binding affinity and T cell response to glucocorticoids. J Immunol. 1993;151:3460-3466. [Cited in This Article: ] |
| 44. | Theriault A, Boyd E, Harrap SB, Hollenberg SM, Connor JM. Regional chromosomal assignment of the human glucocorticoid receptor gene to 5q31. Hum Genet. 1989;83:289-291. [Cited in This Article: ] |
| 45. | Manenschijn L, van den Akker EL, Lamberts SW, van Rossum EF. Clinical features associated with glucocorticoid receptor polymorphisms. An overview. Ann N Y Acad Sci. 2009;1179:179-198. [Cited in This Article: ] |
| 46. | Rosmond R, Chagnon YC, Holm G, Chagnon M, Pérusse L, Lindell K, Carlsson B, Bouchard C, Björntorp P. A glucocorticoid receptor gene marker is associated with abdominal obesity, leptin, and dysregulation of the hypothalamic-pituitary-adrenal axis. Obes Res. 2000;8:211-218. [Cited in This Article: ] |
| 47. | van Rossum EF, Lamberts SW. Polymorphisms in the glucocorticoid receptor gene and their associations with metabolic parameters and body composition. Recent Prog Horm Res. 2004;59:333-357. [Cited in This Article: ] |
| 48. | de Lange P, Koper JW, Huizenga NA, Brinkmann AO, de Jong FH, Karl M, Chrousos GP, Lamberts SW. Differential hormone-dependent transcriptional activation and -repression by naturally occurring human glucocorticoid receptor variants. Mol Endocrinol. 1997;11:1156-1164. [Cited in This Article: ] |
| 49. | Russcher H, Smit P, van den Akker EL, van Rossum EF, Brinkmann AO, de Jong FH, Lamberts SW, Koper JW. Two polymorphisms in the glucocorticoid receptor gene directly affect glucocorticoid-regulated gene expression. J Clin Endocrinol Metab. 2005;90:5804-5810. [Cited in This Article: ] |
| 50. | van Rossum EF, Koper JW, Huizenga NA, Uitterlinden AG, Janssen JA, Brinkmann AO, Grobbee DE, de Jong FH, van Duyn CM, Pols HA. A polymorphism in the glucocorticoid receptor gene, which decreases sensitivity to glucocorticoids in vivo, is associated with low insulin and cholesterol levels. Diabetes. 2002;51:3128-3134. [Cited in This Article: ] |
| 51. | van Rossum EF, Feelders RA, van den Beld AW, Uitterlinden AG, Janssen JA, Ester W, Brinkmann AO, Grobbee DE, de Jong FH, Pols HA. Association of the ER22/23EK polymorphism in the glucocorticoid receptor gene with survival and C-reactive protein levels in elderly men. Am J Med. 2004;117:158-162. [Cited in This Article: ] |
| 52. | Derijk RH, Schaaf MJ, Turner G, Datson NA, Vreugdenhil E, Cidlowski J, de Kloet ER, Emery P, Sternberg EM, Detera-Wadleigh SD. A human glucocorticoid receptor gene variant that increases the stability of the glucocorticoid receptor beta-isoform mRNA is associated with rheumatoid arthritis. J Rheumatol. 2001;28:2383-2388. [Cited in This Article: ] |
| 53. | Hagendorf A, Koper JW, de Jong FH, Brinkmann AO, Lamberts SW, Feelders RA. Expression of the human glucocorticoid receptor splice variants alpha, beta, and P in peripheral blood mononuclear leukocytes in healthy controls and in patients with hyper- and hypocortisolism. J Clin Endocrinol Metab. 2005;90:6237-6243. [Cited in This Article: ] |
| 54. | Lewis-Tuffin LJ, Cidlowski JA. The physiology of human glucocorticoid receptor beta (hGRbeta) and glucocorticoid resistance. Ann N Y Acad Sci. 2006;1069:1-9. [Cited in This Article: ] |
| 55. | Charmandari E, Chrousos GP, Ichijo T, Bhattacharyya N, Vottero A, Souvatzoglou E, Kino T. The human glucocorticoid receptor (hGR) beta isoform suppresses the transcriptional activity of hGRalpha by interfering with formation of active coactivator complexes. Mol Endocrinol. 2005;19:52-64. [Cited in This Article: ] |
| 56. | Charmandari E, Raji A, Kino T, Ichijo T, Tiulpakov A, Zachman K, Chrousos GP. A novel point mutation in the ligand-binding domain (LBD) of the human glucocorticoid receptor (hGR) causing generalized glucocorticoid resistance: the importance of the C terminus of hGR LBD in conferring transactivational activity. J Clin Endocrinol Metab. 2005;90:3696-3705. [Cited in This Article: ] |
| 57. | Oakley RH, Jewell CM, Yudt MR, Bofetiado DM, Cidlowski JA. The dominant negative activity of the human glucocorticoid receptor beta isoform. Specificity and mechanisms of action. J Biol Chem. 1999;274:27857-27866. [Cited in This Article: ] |
| 58. | Oakley RH, Sar M, Cidlowski JA. The human glucocorticoid receptor beta isoform. Expression, biochemical properties, and putative function. J Biol Chem. 1996;271:9550-9559. [Cited in This Article: ] |
| 59. | Honda M, Orii F, Ayabe T, Imai S, Ashida T, Obara T, Kohgo Y. Expression of glucocorticoid receptor beta in lymphocytes of patients with glucocorticoid-resistant ulcerative colitis. Gastroenterology. 2000;118:859-866. [Cited in This Article: ] |
| 60. | Fujishima S, Takeda H, Kawata S, Yamakawa M. The relationship between the expression of the glucocorticoid receptor in biopsied colonic mucosa and the glucocorticoid responsiveness of ulcerative colitis patients. Clin Immunol. 2009;133:208-217. [Cited in This Article: ] |
| 61. | De Iudicibus S, Stocco G, Martelossi S, Drigo I, Norbedo S, Lionetti P, Pozzi E, Barabino A, Decorti G, Bartoli F. Association of BclI polymorphism of the glucocorticoid receptor gene locus with response to glucocorticoids in inflammatory bowel disease. Gut. 2007;56:1319-1320. [Cited in This Article: ] |
| 62. | Oretti C, Marino S, Mosca F, Colnaghi MR, De Iudicibus S, Drigo I, Stocco G, Bartoli F, Decorti G, Demarini S. Glutathione-S-transferase-P1 I105V polymorphism and response to antenatal betamethasone in the prevention of respiratory distress syndrome. Eur J Clin Pharmacol. 2009;65:483-491. [Cited in This Article: ] |
| 63. | Marino S, Verzegnassi F, Tamaro P, Stocco G, Bartoli F, Decorti G, Rabusin M. Response to glucocorticoids and toxicity in childhood acute lymphoblastic leukemia: role of polymorphisms of genes involved in glucocorticoid response. Pediatr Blood Cancer. 2009;53:984-991. [Cited in This Article: ] |
| 64. | van Winsen LM, Manenschijn L, van Rossum EF, Crusius JB, Koper JW, Polman CH, Uitdehaag BM. A glucocorticoid receptor gene haplotype (TthIII1/ER22/23EK/9beta) is associated with a more aggressive disease course in multiple sclerosis. J Clin Endocrinol Metab. 2009;94:2110-2114. [Cited in This Article: ] |
| 65. | van den Akker EL, Russcher H, van Rossum EF, Brinkmann AO, de Jong FH, Hokken A, Pols HA, Koper JW, Lamberts SW. Glucocorticoid receptor polymorphism affects transrepression but not transactivation. J Clin Endocrinol Metab. 2006;91:2800-2803. [Cited in This Article: ] |
| 66. | Huizenga NA, Koper JW, De Lange P, Pols HA, Stolk RP, Burger H, Grobbee DE, Brinkmann AO, De Jong FH, Lamberts SW. A polymorphism in the glucocorticoid receptor gene may be associated with and increased sensitivity to glucocorticoids in vivo. J Clin Endocrinol Metab. 1998;83:144-151. [Cited in This Article: ] |
| 67. | Szabó V, Borgulya G, Filkorn T, Majnik J, Bányász I, Nagy ZZ. The variant N363S of glucocorticoid receptor in steroid-induced ocular hypertension in Hungarian patients treated with photorefractive keratectomy. Mol Vis. 2007;13:659-666. [Cited in This Article: ] |
| 68. | Bonifati DM, Witchel SF, Ermani M, Hoffman EP, Angelini C, Pegoraro E. The glucocorticoid receptor N363S polymorphism and steroid response in Duchenne dystrophy. J Neurol Neurosurg Psychiatry. 2006;77:1177-1179. [Cited in This Article: ] |
| 69. | van Rossum EF, Koper JW, van den Beld AW, Uitterlinden AG, Arp P, Ester W, Janssen JA, Brinkmann AO, de Jong FH, Grobbee DE. Identification of the BclI polymorphism in the glucocorticoid receptor gene: association with sensitivity to glucocorticoids in vivo and body mass index. Clin Endocrinol (Oxf). 2003;59:585-592. [Cited in This Article: ] |
| 70. | Di Blasio AM, van Rossum EF, Maestrini S, Berselli ME, Tagliaferri M, Podestà F, Koper JW, Liuzzi A, Lamberts SW. The relation between two polymorphisms in the glucocorticoid receptor gene and body mass index, blood pressure and cholesterol in obese patients. Clin Endocrinol (Oxf). 2003;59:68-74. [Cited in This Article: ] |
| 71. | Panarelli M, Holloway CD, Fraser R, Connell JM, Ingram MC, Anderson NH, Kenyon CJ. Glucocorticoid receptor polymorphism, skin vasoconstriction, and other metabolic intermediate phenotypes in normal human subjects. J Clin Endocrinol Metab. 1998;83:1846-1852. [Cited in This Article: ] |
| 72. | De Iudicibus S, Stocco G, Martelossi S, Londero M, Ebner E, Pontillo A, Lionetti P, Barabino A, Bartoli F, Ventura A. Genetic predictors of glucocorticoid response in pediatric patients with inflammatory bowel diseases. J Clin Gastroenterol. 2011;45:e1-e7. [Cited in This Article: ] |
| 73. | Gross KL, Lu NZ, Cidlowski JA. Molecular mechanisms regulating glucocorticoid sensitivity and resistance. Mol Cell Endocrinol. 2009;300:7-16. [Cited in This Article: ] |
| 74. | Qian X, Zhu Y, Xu W, Lin Y. Glucocorticoid receptor and heat shock protein 90 in peripheral blood mononuclear cells from asthmatics. Chin Med J (Engl). 2001;114:1051-1054. [Cited in This Article: ] |
| 75. | Matysiak M, Makosa B, Walczak A, Selmaj K. Patients with multiple sclerosis resisted to glucocorticoid therapy: abnormal expression of heat-shock protein 90 in glucocorticoid receptor complex. Mult Scler. 2008;14:919-926. [Cited in This Article: ] |
| 76. | Ouyang J, Jiang T, Tan M, Cui Y, Li X. Abnormal expression and distribution of heat shock protein 90: potential etiologic immunoendocrine mechanism of glucocorticoid resistance in idiopathic nephrotic syndrome. Clin Vaccine Immunol. 2006;13:496-500. [Cited in This Article: ] |
| 77. | Kojika S, Sugita K, Inukai T, Saito M, Iijima K, Tezuka T, Goi K, Shiraishi K, Mori T, Okazaki T. Mechanisms of glucocorticoid resistance in human leukemic cells: implication of abnormal 90 and 70 kDa heat shock proteins. Leukemia. 1996;10:994-999. [Cited in This Article: ] |
| 78. | Lauten M, Cario G, Asgedom G, Welte K, Schrappe M. Protein expression of the glucocorticoid receptor in childhood acute lymphoblastic leukemia. Haematologica. 2003;88:1253-1258. [Cited in This Article: ] |
| 79. | Hawkins GA, Lazarus R, Smith RS, Tantisira KG, Meyers DA, Peters SP, Weiss ST, Bleecker ER. The glucocorticoid receptor heterocomplex gene STIP1 is associated with improved lung function in asthmatic subjects treated with inhaled corticosteroids. J Allergy Clin Immunol. 2009;123:1376-1383.e7. [Cited in This Article: ] |
| 80. | Denny WB, Valentine DL, Reynolds PD, Smith DF, Scammell JG. Squirrel monkey immunophilin FKBP51 is a potent inhibitor of glucocorticoid receptor binding. Endocrinology. 2000;141:4107-4113. [Cited in This Article: ] |
| 81. | Riggs DL, Roberts PJ, Chirillo SC, Cheung-Flynn J, Prapapanich V, Ratajczak T, Gaber R, Picard D, Smith DF. The Hsp90-binding peptidylprolyl isomerase FKBP52 potentiates glucocorticoid signaling in vivo. EMBO J. 2003;22:1158-1167. [Cited in This Article: ] |
| 82. | Wochnik GM, Rüegg J, Abel GA, Schmidt U, Holsboer F, Rein T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J Biol Chem. 2005;280:4609-4616. [Cited in This Article: ] |
| 83. | Zhang C, Sweezey NB, Gagnon S, Muskat B, Koehler D, Post M, Kaplan F. A novel karyopherin-beta homolog is developmentally and hormonally regulated in fetal lung. Am J Respir Cell Mol Biol. 2000;22:451-459. [Cited in This Article: ] |
| 84. | Tao T, Lan J, Lukacs GL, Haché RJ, Kaplan F. Importin 13 regulates nuclear import of the glucocorticoid receptor in airway epithelial cells. Am J Respir Cell Mol Biol. 2006;35:668-680. [Cited in This Article: ] |
| 85. | Mingot JM, Kostka S, Kraft R, Hartmann E, Görlich D. Importin 13: a novel mediator of nuclear import and export. EMBO J. 2001;20:3685-3694. [Cited in This Article: ] |
| 86. | Raby BA, Van Steen K, Lasky-Su J, Tantisira K, Kaplan F, Weiss ST. Importin-13 genetic variation is associated with improved airway responsiveness in childhood asthma. Respir Res. 2009;10:67. [Cited in This Article: ] |
| 87. | Percipalle P, Farrants AK. Chromatin remodelling and transcription: be-WICHed by nuclear myosin 1. Curr Opin Cell Biol. 2006;18:267-274. [Cited in This Article: ] |
| 88. | Narlikar GJ, Fan HY, Kingston RE. Cooperation between complexes that regulate chromatin structure and transcription. Cell. 2002;108:475-487. [Cited in This Article: ] |
| 89. | de la Serna IL, Ohkawa Y, Imbalzano AN. Chromatin remodelling in mammalian differentiation: lessons from ATP-dependent remodellers. Nat Rev Genet. 2006;7:461-473. [Cited in This Article: ] |
| 90. | Becker PB, Hörz W. ATP-dependent nucleosome remodeling. Annu Rev Biochem. 2002;71:247-273. [Cited in This Article: ] |
| 91. | Yoshinaga SK, Peterson CL, Herskowitz I, Yamamoto KR. Roles of SWI1, SWI2, and SWI3 proteins for transcriptional enhancement by steroid receptors. Science. 1992;258:1598-1604. [Cited in This Article: ] |
| 92. | Wallberg AE, Neely KE, Hassan AH, Gustafsson JA, Workman JL, Wright AP. Recruitment of the SWI-SNF chromatin remodeling complex as a mechanism of gene activation by the glucocorticoid receptor tau1 activation domain. Mol Cell Biol. 2000;20:2004-2013. [Cited in This Article: ] |
| 93. | Ostlund Farrants AK, Blomquist P, Kwon H, Wrange O. Glucocorticoid receptor-glucocorticoid response element binding stimulates nucleosome disruption by the SWI/SNF complex. Mol Cell Biol. 1997;17:895-905. [Cited in This Article: ] |
| 94. | Wade PA, Wolffe AP. Transcriptional regulation: SWItching circuitry. Curr Biol. 1999;9:R221-R224. [Cited in This Article: ] |
| 95. | Roberts CW, Orkin SH. The SWI/SNF complex--chromatin and cancer. Nat Rev Cancer. 2004;4:133-142. [Cited in This Article: ] |
| 96. | Klochendler-Yeivin A, Muchardt C, Yaniv M. SWI/SNF chromatin remodeling and cancer. Curr Opin Genet Dev. 2002;12:73-79. [Cited in This Article: ] |
| 97. | Holleman A, Cheok MH, den Boer ML, Yang W, Veerman AJ, Kazemier KM, Pei D, Cheng C, Pui CH, Relling MV. Gene-expression patterns in drug-resistant acute lymphoblastic leukemia cells and response to treatment. N Engl J Med. 2004;351:533-542. [Cited in This Article: ] |
| 98. | Pottier N, Yang W, Assem M, Panetta JC, Pei D, Paugh SW, Cheng C, Den Boer ML, Relling MV, Pieters R. The SWI/SNF chromatin-remodeling complex and glucocorticoid resistance in acute lymphoblastic leukemia. J Natl Cancer Inst. 2008;100:1792-1803. [Cited in This Article: ] |
| 99. | Pottier N, Cheok MH, Yang W, Assem M, Tracey L, Obenauer JC, Panetta JC, Relling MV, Evans WE. Expression of SMARCB1 modulates steroid sensitivity in human lymphoblastoid cells: identification of a promoter SNP that alters PARP1 binding and SMARCB1 expression. Hum Mol Genet. 2007;16:2261-2271. [Cited in This Article: ] |
| 100. | Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55-89. [Cited in This Article: ] |
| 101. | Dhabhar FS, McEwen BS. Enhancing versus suppressive effects of stress hormones on skin immune function. Proc Natl Acad Sci USA. 1999;96:1059-1064. [Cited in This Article: ] |
| 102. | Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351-1362. [Cited in This Article: ] |
| 103. | Charmandari E, Kino T, Chrousos GP. Glucocorticoids and their actions: an introduction. Ann N Y Acad Sci. 2004;1024:1-8. [Cited in This Article: ] |
| 104. | Galon J, Franchimont D, Hiroi N, Frey G, Boettner A, Ehrhart-Bornstein M, O'Shea JJ, Chrousos GP, Bornstein SR. Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. FASEB J. 2002;16:61-71. [Cited in This Article: ] |
| 105. | Ashwell JD, Lu FW, Vacchio MS. Glucocorticoids in T cell development and function*. Annu Rev Immunol. 2000;18:309-345. [Cited in This Article: ] |
| 106. | Jahnsen FL, Haye R, Gran E, Brandtzaeg P, Johansen FE. Glucocorticosteroids inhibit mRNA expression for eotaxin, eotaxin-2, and monocyte-chemotactic protein-4 in human airway inflammation with eosinophilia. J Immunol. 1999;163:1545-1551. [Cited in This Article: ] |
| 107. | Pype JL, Dupont LJ, Menten P, Van Coillie E, Opdenakker G, Van Damme J, Chung KF, Demedts MG, Verleden GM. Expression of monocyte chemotactic protein (MCP)-1, MCP-2, and MCP-3 by human airway smooth-muscle cells. Modulation by corticosteroids and T-helper 2 cytokines. Am J Respir Cell Mol Biol. 1999;21:528-536. [Cited in This Article: ] |
| 108. | Bioque G, Crusius JB, Koutroubakis I, Bouma G, Kostense PJ, Meuwissen SG, Peña AS. Allelic polymorphism in IL-1 beta and IL-1 receptor antagonist (IL-1Ra) genes in inflammatory bowel disease. Clin Exp Immunol. 1995;102:379-383. [Cited in This Article: ] |
| 109. | Cantagrel A, Navaux F, Loubet-Lescoulié P, Nourhashemi F, Enault G, Abbal M, Constantin A, Laroche M, Mazières B. Interleukin-1beta, interleukin-1 receptor antagonist, interleukin-4, and interleukin-10 gene polymorphisms: relationship to occurrence and severity of rheumatoid arthritis. Arthritis Rheum. 1999;42:1093-1100. [Cited in This Article: ] |
| 110. | Apte RN, Dotan S, Elkabets M, White MR, Reich E, Carmi Y, Song X, Dvozkin T, Krelin Y, Voronov E. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev. 2006;25:387-408. [Cited in This Article: ] |
| 111. | Buttgereit F, Saag KG, Cutolo M, da Silva JA, Bijlsma JW. The molecular basis for the effectiveness, toxicity, and resistance to glucocorticoids: focus on the treatment of rheumatoid arthritis. Scand J Rheumatol. 2005;34:14-21. [Cited in This Article: ] |
| 112. | Barnes PJ, Adcock IM. Glucocorticoid resistance in inflammatory diseases. Lancet. 2009;373:1905-1917. [Cited in This Article: ] |
| 113. | Farrell RJ, Kelleher D. Glucocorticoid resistance in inflammatory bowel disease. J Endocrinol. 2003;178:339-346. [Cited in This Article: ] |
| 114. | Miller AH, Pariante CM, Pearce BD. Effects of cytokines on glucocorticoid receptor expression and function. Glucocorticoid resistance and relevance to depression. Adv Exp Med Biol. 1999;461:107-116. [Cited in This Article: ] |
| 115. | Batrakova EV, Li S, Miller DW, Kabanov AV. Pluronic P85 increases permeability of a broad spectrum of drugs in polarized BBMEC and Caco-2 cell monolayers. Pharm Res. 1999;16:1366-1372. [Cited in This Article: ] |
| 116. | Hill MR, Stith RD, McCallum RE. Human recombinant IL-1 alters glucocorticoid receptor function in Reuber hepatoma cells. J Immunol. 1988;141:1522-1528. [Cited in This Article: ] |
| 117. | Raddatz D, Toth S, Schwörer H, Ramadori G. Glucocorticoid receptor signaling in the intestinal epithelial cell lines IEC-6 and Caco-2: evidence of inhibition by interleukin-1beta. Int J Colorectal Dis. 2001;16:377-383. [Cited in This Article: ] |
| 118. | Franchimont D, Martens H, Hagelstein MT, Louis E, Dewe W, Chrousos GP, Belaiche J, Geenen V. Tumor necrosis factor alpha decreases, and interleukin-10 increases, the sensitivity of human monocytes to dexamethasone: potential regulation of the glucocorticoid receptor. J Clin Endocrinol Metab. 1999;84:2834-2839. [Cited in This Article: ] |
| 119. | Ishiguro Y. Mucosal proinflammatory cytokine production correlates with endoscopic activity of ulcerative colitis. J Gastroenterol. 1999;34:66-74. [Cited in This Article: ] |
| 120. | Leung DY, Hamid Q, Vottero A, Szefler SJ, Surs W, Minshall E, Chrousos GP, Klemm DJ. Association of glucocorticoid insensitivity with increased expression of glucocorticoid receptor beta. J Exp Med. 1997;186:1567-1574. [Cited in This Article: ] |
| 121. | Goleva E, Kisich KO, Leung DY. A role for STAT5 in the pathogenesis of IL-2-induced glucocorticoid resistance. J Immunol. 2002;169:5934-5940. [Cited in This Article: ] |
| 122. | Adcock IM, Lane SJ, Brown CR, Lee TH, Barnes PJ. Abnormal glucocorticoid receptor-activator protein 1 interaction in steroid-resistant asthma. J Exp Med. 1995;182:1951-1958. [Cited in This Article: ] |
| 123. | Irusen E, Matthews JG, Takahashi A, Barnes PJ, Chung KF, Adcock IM. p38 Mitogen-activated protein kinase-induced glucocorticoid receptor phosphorylation reduces its activity: role in steroid-insensitive asthma. J Allergy Clin Immunol. 2002;109:649-657. [Cited in This Article: ] |
| 124. | Clark AR. MAP kinase phosphatase 1: a novel mediator of biological effects of glucocorticoids? J Endocrinol. 2003;178:5-12. [Cited in This Article: ] |
| 125. | Angst E, Reber HA, Hines OJ, Eibl G. Mononuclear cell-derived interleukin-1 beta confers chemoresistance in pancreatic cancer cells by upregulation of cyclooxygenase-2. Surgery. 2008;144:57-65. [Cited in This Article: ] |
| 126. | Akira S, Hirano T, Taga T, Kishimoto T. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF). FASEB J. 1990;4:2860-2867. [Cited in This Article: ] |
| 127. | Dinarello CA. Blocking IL-1 in systemic inflammation. J Exp Med. 2005;201:1355-1359. [Cited in This Article: ] |
| 128. | Nicklin MJ, Weith A, Duff GW. A physical map of the region encompassing the human interleukin-1 alpha, interleukin-1 beta, and interleukin-1 receptor antagonist genes. Genomics. 1994;19:382-384. [Cited in This Article: ] |
| 129. | Webb AC, Collins KL, Auron PE, Eddy RL, Nakai H, Byers MG, Haley LL, Henry WM, Shows TB. Interleukin-1 gene (IL1) assigned to long arm of human chromosome 2. Lymphokine Res. 1986;5:77-85. [Cited in This Article: ] |
| 130. | Haukim N, Bidwell JL, Smith AJ, Keen LJ, Gallagher G, Kimberly R, Huizinga T, McDermott MF, Oksenberg J, McNicholl J. Cytokine gene polymorphism in human disease: on-line databases, supplement 2. Genes Immun. 2002;3:313-330. [Cited in This Article: ] |
| 131. | Pociot F, Mølvig J, Wogensen L, Worsaae H, Nerup J. A TaqI polymorphism in the human interleukin-1 beta (IL-1 beta) gene correlates with IL-1 beta secretion in vitro. Eur J Clin Invest. 1992;22:396-402. [Cited in This Article: ] |
| 132. | Hall SK, Perregaux DG, Gabel CA, Woodworth T, Durham LK, Huizinga TW, Breedveld FC, Seymour AB. Correlation of polymorphic variation in the promoter region of the interleukin-1 beta gene with secretion of interleukin-1 beta protein. Arthritis Rheum. 2004;50:1976-1983. [Cited in This Article: ] |
| 133. | Dominici R, Malferrari G, Mariani C, Grimaldi L, Biunno I. The Interleukin 1-beta exonic (+3953) polymorphism does not alter in vitro protein secretion. Exp Mol Pathol. 2002;73:139-141. [Cited in This Article: ] |
| 134. | Maury CP, Liljeström M, Laiho K, Tiitinen S, Kaarela K, Hurme M. Anaemia of chronic disease in AA amyloidosis is associated with allele 2 of the interleukin-1beta-511 promoter gene and raised levels of interleukin-1beta and interleukin-18. J Intern Med. 2004;256:145-152. [Cited in This Article: ] |
| 135. | Guasch JF, Bertina RM, Reitsma PH. Five novel intragenic dimorphisms in the human interleukin-1 genes combine to high informativity. Cytokine. 1996;8:598-602. [Cited in This Article: ] |
| 136. | Markova S, Nakamura T, Makimoto H, Ichijima T, Yamamori M, Kuwahara A, Iwaki K, Nishiguchi K, Okamura N, Okumura K. IL-1beta genotype-related effect of prednisolone on IL-1beta production in human peripheral blood mononuclear cells under acute inflammation. Biol Pharm Bull. 2007;30:1481-1487. [Cited in This Article: ] |
| 137. | Hamacher R, Diersch S, Scheibel M, Eckel F, Mayr M, Rad R, Bajbouj M, Schmid RM, Saur D, Schneider G. Interleukin 1 beta gene promoter SNPs are associated with risk of pancreatic cancer. Cytokine. 2009;46:182-186. [Cited in This Article: ] |
| 138. | Kimura R, Nishioka T, Soemantri A, Ishida T. Cis-acting effect of the IL1B C-31T polymorphism on IL-1 beta mRNA expression. Genes Immun. 2004;5:572-575. [Cited in This Article: ] |
| 139. | Cerretti DP, Kozlosky CJ, Mosley B, Nelson N, Van Ness K, Greenstreet TA, March CJ, Kronheim SR, Druck T, Cannizzaro LA. Molecular cloning of the interleukin-1 beta converting enzyme. Science. 1992;256:97-100. [Cited in This Article: ] |
| 140. | Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356:768-774. [Cited in This Article: ] |
| 141. | Jin Y, Birlea SA, Fain PR, Spritz RA. Genetic variations in NALP1 are associated with generalized vitiligo in a Romanian population. J Invest Dermatol. 2007;127:2558-2562. [Cited in This Article: ] |
| 142. | Jin Y, Mailloux CM, Gowan K, Riccardi SL, LaBerge G, Bennett DC, Fain PR, Spritz RA. NALP1 in vitiligo-associated multiple autoimmune disease. N Engl J Med. 2007;356:1216-1225. [Cited in This Article: ] |
| 143. | Magitta NF, Bøe Wolff AS, Johansson S, Skinningsrud B, Lie BA, Myhr KM, Undlien DE, Joner G, Njølstad PR, Kvien TK. A coding polymorphism in NALP1 confers risk for autoimmune Addison's disease and type 1 diabetes. Genes Immun. 2009;10:120-124. [Cited in This Article: ] |
| 144. | Tracey KJ, Cerami A. Tumor necrosis factor: a pleiotropic cytokine and therapeutic target. Annu Rev Med. 1994;45:491-503. [Cited in This Article: ] |
| 145. | Carroll MC, Katzman P, Alicot EM, Koller BH, Geraghty DE, Orr HT, Strominger JL, Spies T. Linkage map of the human major histocompatibility complex including the tumor necrosis factor genes. Proc Natl Acad Sci USA. 1987;84:8535-8539. [Cited in This Article: ] |
| 146. | Harney SM, Newton JL, Wordsworth BP. Molecular genetics of rheumatoid arthritis. Curr Opin Pharmacol. 2003;3:280-285. [Cited in This Article: ] |
| 147. | Elahi MM, Asotra K, Matata BM, Mastana SS. Tumor necrosis factor alpha -308 gene locus promoter polymorphism: an analysis of association with health and disease. Biochim Biophys Acta. 2009;1792:163-172. [Cited in This Article: ] |
| 148. | Wilson AG, de Vries N, Pociot F, di Giovine FS, van der Putte LB, Duff GW. An allelic polymorphism within the human tumor necrosis factor alpha promoter region is strongly associated with HLA A1, B8, and DR3 alleles. J Exp Med. 1993;177:557-560. [Cited in This Article: ] |
| 149. | McManus R, Wilson AG, Mansfield J, Weir DG, Duff GW, Kelleher D. TNF2, a polymorphism of the tumour necrosis-alpha gene promoter, is a component of the celiac disease major histocompatibility complex haplotype. Eur J Immunol. 1996;26:2113-2118. [Cited in This Article: ] |
| 150. | Candore G, Lio D, Colonna Romano G, Caruso C. Pathogenesis of autoimmune diseases associated with 8.1 ancestral haplotype: effect of multiple gene interactions. Autoimmun Rev. 2002;1:29-35. [Cited in This Article: ] |
| 151. | Candore G, Balistreri CR, Colonna-Romano G, Lio D, Caruso C. Major histocompatibility complex and sporadic Alzheimer's disease: a critical reappraisal. Exp Gerontol. 2004;39:645-652. [Cited in This Article: ] |
| 152. | Cucchiara S, Latiano A, Palmieri O, Canani RB, D'Incà R, Guariso G, Vieni G, De Venuto D, Riegler G, De'Angelis GL. Polymorphisms of tumor necrosis factor-alpha but not MDR1 influence response to medical therapy in pediatric-onset inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2007;44:171-179. [Cited in This Article: ] |
| 153. | Li Kam Wa TC, Mansur AH, Britton J, Williams G, Pavord I, Richards K, Campbell DA, Morton N, Holgate ST, Morrison JF. Association between - 308 tumour necrosis factor promoter polymorphism and bronchial hyperreactivity in asthma. Clin Exp Allergy. 1999;29:1204-1208. [Cited in This Article: ] |
| 154. | Cvetkovic JT, Wallberg-Jonsson S, Stegmayr B, Rantapaa-Dahlqvist S, Lefvert AK. Susceptibility for and clinical manifestations of rheumatoid arthritis are associated with polymorphisms of the TNF-alpha, IL-1beta, and IL-1Ra genes. J Rheumatol. 2002;29:212-219. [Cited in This Article: ] |
| 155. | Louis E, Peeters M, Franchimont D, Seidel L, Fontaine F, Demolin G, Croes F, Dupont P, Davin L, Omri S. Tumour necrosis factor (TNF) gene polymorphism in Crohn's disease (CD): influence on disease behaviour? Clin Exp Immunol. 2000;119:64-68. [Cited in This Article: ] |
| 156. | Tripathi G, Jafar T, Mandal K, Mahdi AA, Awasthi S, Sharma RK, Kumar A, Gulati S, Agrawal S. Does cytokine gene polymorphism affect steroid responses in idiopathic nephrotic syndrome? Indian J Med Sci. 2008;62:383-391. [Cited in This Article: ] |
| 157. | Sankaran D, Asderakis A, Ashraf S, Roberts IS, Short CD, Dyer PA, Sinnott PJ, Hutchinson IV. Cytokine gene polymorphisms predict acute graft rejection following renal transplantation. Kidney Int. 1999;56:281-288. [Cited in This Article: ] |
| 158. | Warzocha K, Ribeiro P, Bienvenu J, Roy P, Charlot C, Rigal D, Coiffier B, Salles G. Genetic polymorphisms in the tumor necrosis factor locus influence non-Hodgkin's lymphoma outcome. Blood. 1998;91:3574-3581. [Cited in This Article: ] |
| 159. | Lauten M, Matthias T, Stanulla M, Beger C, Welte K, Schrappe M. Association of initial response to prednisone treatment in childhood acute lymphoblastic leukaemia and polymorphisms within the tumour necrosis factor and the interleukin-10 genes. Leukemia. 2002;16:1437-1442. [Cited in This Article: ] |
| 160. | Khatri VP, Caligiuri MA. A review of the association between interleukin-10 and human B-cell malignancies. Cancer Immunol Immunother. 1998;46:239-244. [Cited in This Article: ] |
| 161. | Klein B, Lu ZY, Gu ZJ, Costes V, Jourdan M, Rossi JF. Interleukin-10 and Gp130 cytokines in human multiple myeloma. Leuk Lymphoma. 1999;34:63-70. [Cited in This Article: ] |
| 162. | Franchimont D. Overview of the actions of glucocorticoids on the immune response: a good model to characterize new pathways of immunosuppression for new treatment strategies. Ann N Y Acad Sci. 2004;1024:124-137. [Cited in This Article: ] |
| 163. | Middleton PG, Taylor PR, Jackson G, Proctor SJ, Dickinson AM. Cytokine gene polymorphisms associating with severe acute graft-versus-host disease in HLA-identical sibling transplants. Blood. 1998;92:3943-3948. [Cited in This Article: ] |
| 164. | de Paz B, Alperi-López M, Ballina-García FJ, Prado C, Mozo L, Gutiérrez C, Suárez A. Interleukin 10 and tumor necrosis factor-alpha genotypes in rheumatoid arthritis--association with clinical response to glucocorticoids. J Rheumatol. 2010;37:503-511. [Cited in This Article: ] |
| 165. | Higgins CF, Callaghan R, Linton KJ, Rosenberg MF, Ford RC. Structure of the multidrug resistance P-glycoprotein. Semin Cancer Biol. 1997;8:135-142. [Cited in This Article: ] |
| 166. | Farrell RJ, Menconi MJ, Keates AC, Kelly CP. P-glycoprotein-170 inhibition significantly reduces cortisol and ciclosporin efflux from human intestinal epithelial cells and T lymphocytes. Aliment Pharmacol Ther. 2002;16:1021-1031. [Cited in This Article: ] |
| 167. | Farrell RJ, Murphy A, Long A, Donnelly S, Cherikuri A, O'Toole D, Mahmud N, Keeling PW, Weir DG, Kelleher D. High multidrug resistance (P-glycoprotein 170) expression in inflammatory bowel disease patients who fail medical therapy. Gastroenterology. 2000;118:279-288. [Cited in This Article: ] |
| 168. | Hirano T, Onda K, Toma T, Miyaoka M, Moriyasu F, Oka K. MDR1 mRNA expressions in peripheral blood mononuclear cells of patients with ulcerative colitis in relation to glucocorticoid administration. J Clin Pharmacol. 2004;44:481-486. [Cited in This Article: ] |
| 169. | Callen DF, Baker E, Simmers RN, Seshadri R, Roninson IB. Localization of the human multiple drug resistance gene, MDR1, to 7q21.1. Hum Genet. 1987;77:142-144. [Cited in This Article: ] |
| 170. | Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmöller J, Johne A, Cascorbi I, Gerloff T, Roots I, Eichelbaum M. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci USA. 2000;97:3473-3478. [Cited in This Article: ] |
| 171. | Cascorbi I, Gerloff T, Johne A, Meisel C, Hoffmeyer S, Schwab M, Schaeffeler E, Eichelbaum M, Brinkmann U, Roots I. Frequency of single nucleotide polymorphisms in the P-glycoprotein drug transporter MDR1 gene in white subjects. Clin Pharmacol Ther. 2001;69:169-174. [Cited in This Article: ] |
| 172. | Kim RB, Leake BF, Choo EF, Dresser GK, Kubba SV, Schwarz UI, Taylor A, Xie HG, McKinsey J, Zhou S. Identification of functionally variant MDR1 alleles among European Americans and African Americans. Clin Pharmacol Ther. 2001;70:189-199. [Cited in This Article: ] |
| 173. | Potocnik U, Ferkolj I, Glavac D, Dean M. Polymorphisms in multidrug resistance 1 (MDR1) gene are associated with refractory Crohn disease and ulcerative colitis. Genes Immun. 2004;5:530-539. [Cited in This Article: ] |
| 174. | Palmieri O, Latiano A, Valvano R, D'Incà R, Vecchi M, Sturniolo GC, Saibeni S, Bossa F, Latiano T, Devoto M. Multidrug resistance 1 gene polymorphisms are not associated with inflammatory bowel disease and response to therapy in Italian patients. Aliment Pharmacol Ther. 2005;22:1129-1138. [Cited in This Article: ] |
| 175. | Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, Watkins PB, Daly A, Wrighton SA, Hall SD. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001;27:383-391. [Cited in This Article: ] |
| 176. | Pichard L, Fabre I, Daujat M, Domergue J, Joyeux H, Maurel P. Effect of corticosteroids on the expression of cytochromes P450 and on cyclosporin A oxidase activity in primary cultures of human hepatocytes. Mol Pharmacol. 1992;41:1047-1055. [Cited in This Article: ] |
| 177. | Wilkinson GR. Drug metabolism and variability among patients in drug response. N Engl J Med. 2005;352:2211-2221. [Cited in This Article: ] |
| 178. | Hustert E, Haberl M, Burk O, Wolbold R, He YQ, Klein K, Nuessler AC, Neuhaus P, Klattig J, Eiselt R. The genetic determinants of the CYP3A5 polymorphism. Pharmacogenetics. 2001;11:773-779. [Cited in This Article: ] |
| 179. | Chou FC, Tzeng SJ, Huang JD. Genetic polymorphism of cytochrome P450 3A5 in Chinese. Drug Metab Dispos. 2001;29:1205-1209. [Cited in This Article: ] |
| 180. | Miura M, Satoh S, Inoue K, Kagaya H, Saito M, Inoue T, Habuchi T, Suzuki T. Influence of CYP3A5, ABCB1 and NR1I2 polymorphisms on prednisolone pharmacokinetics in renal transplant recipients. Steroids. 2008;73:1052-1059. [Cited in This Article: ] |
| 181. | Zheng HX, Webber SA, Zeevi A, Schuetz E, Zhang J, Lamba J, Boyle GJ, Wilson JW, Burckart GJ. The impact of pharmacogenomic factors on steroid dependency in pediatric heart transplant patients using logistic regression analysis. Pediatr Transplant. 2004;8:551-557. [Cited in This Article: ] |