Published online Dec 14, 2011. doi: 10.3748/wjg.v17.i46.5123
Revised: March 29, 2011
Accepted: April 5, 2011
Published online: December 14, 2011
AIM: To compare and evaluate the appropriate prognostic indicators of lymph node basic staging in gastric cancer patients who underwent radical resection.
METHODS: A total of 1042 gastric cancer patients who underwent radical resection and D2 lymphadenectomy were staged using the 6th and 7th edition International Union Against Cancer (UICC) N staging methods and the metastatic lymph node ratio (MLNR) staging. Homogeneity, discriminatory ability, and gradient monotonicity of the various staging methods were compared using linear trend χ2, likelihood ratio χ2 statistics, and Akaike information criterion (AIC) calculations. The area under the curve (AUC) was calculated to compare the predictive ability of the aforementioned three staging methods.
RESULTS: Optimal cut-points of the MLNR were calculated as MLNR0 (0), MLNR1 (0.01-0.30), MLNR2 (0.31-0.50), and MLNR3 (0.51-1.00). In univariate, multivariate, and stratified analyses, MLNR staging was superior to the 6th and 7th edition UICC N staging methods. MLNR staging had a higher AUC, higher linear trend and likelihood ratio χ2 scores and lower AIC values than the other two staging methods.
CONCLUSION: MLNR staging predicts survival after gastric cancer more precisely than the 6th and 7th edition UICC N classifications and should be considered as an alternative to current pathological N staging.
- Citation: Xiao LB, Yu JX, Wu WH, Xu FF, Yang SB. Superiority of metastatic lymph node ratio to the 7th edition UICC N staging in gastric cancer. World J Gastroenterol 2011; 17(46): 5123-5130
- URL: https://www.wjgnet.com/1007-9327/full/v17/i46/5123.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i46.5123
In recent years, more cases of gastric cancer have been diagnosed in China than in any other country[1]. Accurate prognosis prediction for gastric cancer patients enables doctors to determine the patients’ expected clinical courses and to have more information when deciding whether to use adjuvant therapy and when comparing the therapeutic effects of different treatment modalities. A widely used classification proposed by the International Union Against Cancer (UICC), the tumor-node-metastasis (TNM) system, combines the most powerful and reliable factors for analyzing tumor status[2,3]. Lymph node metastasis is one of the most important gastric cancer prognostic factors[4]. The identified number of involved lymph nodes depends on the number of lymph nodes removed and examined, which in turn depends on the surgical and pathologic procedures. Although TNM classification is a convenient and reproducible method for precise staging, it demands the examination of at least 15 lymph nodes. If the number of dissected and examined lymph nodes is small, downmigration of N stage may occur, and conversely, if the number is large, upmigration of N stage may occur, which is also referred to as stage migration in some references[5-10]. To improve prognosis prediction, the number of positive lymph nodes should be considered in the context of the number of nodes examined. The metastatic lymph node ratio (MLNR), defined as the number of positive lymph nodes divided by the number of lymph nodes retrieved, has been proposed as an alternative to classification systems that assess the absolute number of positive lymph nodes, such as the UICC (2002, 6th edition) or Japanese Gastric Cancer Association (JGCA) (1998, 2nd English edition) staging systems[11-16].
This year, gastric cancer lymph node metastasis staging was changed in both the UICC 7th edition and the JGCA 14th edition staging systems in that it now depends solely on the number of metastatic nodes found[3,17]. In the new UICC and JGCA systems, patients with one to two positive lymph nodes are classified as N1, patients with three to six positive lymph nodes are classified as N2, and patients with seven or more positive lymph nodes are classified as N3. Some authors have demonstrated that the 7th edition UICC staging system is superior to the 6th edition based on its homogeneity, discriminatory ability and prognostic value[18-20].
However, to date there has been no formal study that focused on comparing the prognostic significance of the MLNR with that of the 7th edition UICC N staging system. In the present article, we investigate whether patients with gastric cancer can be classified into meaningful risk categories based on MLNR by comparing this staging system with the 7th edition UICC N staging system.
Between January 1996 and December 2007, 1042 patients with histologically diagnosed gastric cancer underwent surgery at the Department of Gastrointestinal-pancreatic Surgery, First Affiliated Hospital, Sun Yat-Sen University, China. The postoperative pathological results included tumor size, histological type, margin, adjacent tissues and neighboring organs, lymphatic/venous invasion, retrieved lymph nodes, metastatic lymph nodes, and pTNM staging. The inclusion criteria of the study were as follows: (1) gastric adenocarcinoma identified by histo-pathological examination; (2) histologically confirmed R0 resection, which was defined as no macroscopic or microscopic residual tumor; and (3) availability of complete follow-up data. Patients with distant metastases, a history of familial malignancy or other synchronous malignancy (such as gastrointestinal stromal tumor, esophageal cancer, colorectal cancer, etc.), or carcinoma of the gastric stump after gastric resection for benign disease or who died in the perioperative period were excluded from the study.
D2 lymphadenectomy was performed by experienced surgeons following the JGCA guidelines[21]. A total of 15 313 lymph nodes were retrieved, with a mean of 14.70 ± 10.25 lymph nodes per patient (25.14 ± 9.28 for patients with > 15 lymph nodes retrieved and 8.58 ± 3.87 for patients with ≤ 15 lymph nodes retrieved) and a range from 3 to 66. The mean number of lymph nodes with evidence of metastasis was 6.40 ± 6.90 per patient (9.78 ± 9.42 for patients with > 15 lymph nodes retrieved and 4.15 ± 2.83 for patients with ≤ 15 lymph nodes retrieved), with a range from 1 to 70. Lymph node involvement was classified according to the 7th edition UICC (2010) N staging system (N0: no metastasis; N1: 1-2 metastatic lymph nodes; N2: 3-6 metastatic lymph nodes; N3: ≥ 7 metastatic lymph nodes) and 6th edition UICC (2002) N staging system (N0: no metastasis; N1: 1-6 metastatic lymph nodes; N2: 7-15 metastatic lymph nodes; N3: ≥ 16 metastatic lymph nodes). All nodal material was separately dissected from the specimen by a surgeon at the end of the procedure. Our study does not include stage IV patients, graded according to the UICC 7th edition staging system, because all of the patients enrolled underwent radical resection and had no distant metastasis.
Postoperative follow-up at our outpatient department included clinical and laboratory examinations every 3 mo for the first 2 years, every 6 mo during the third to fifth years, and annually thereafter until at least 5 years after the operation or until the patient died, whichever came first. Overall patient survival, defined as the time from operation to death or final follow-up, was used as a measure of prognosis. The median follow-up for the entire cohort was 56 mo (range 3-178 mo).
To determine the appropriate MLNR cut-points in the entire cohort, our analysis for the best cut-points was conducted as follows: In the first step, we evaluated the prognostic value of the MLNR, adjusting for other clinicopathological covariates that are significantly associated with gastric cancer mortality. Second, patients having no involved lymph nodes (MLNR = 0) were assigned to one group because it has been well documented that their prognosis significantly differs from patients with metastatic lymph nodes[12,13,22,23]. After ascertaining that the MLNR was significantly associated with gastric cancer mortality, we determined two additional appropriate cut-points for categorizing the MLNR to make our cut-points comparable with those for UICC N staging. For this, we recomputed the likelihood associated with all possible pairs of MLNR cutoffs ranging from 0.05 to 0.95 at intervals of 0.05. In our study, the two alternative cut-points for the MLNR were 0.30 and 0.50. Martingale residual analysis was also used to examine the function form of the MLNR, and our cut-points (0, 0.30 and 0.50) were found to be consistent. After extensive evaluations of our data, no other sets of cut-points performed better than those already described. Thus, four subgroups of the MLNR classification (MLNR0, 0%; MLNR1, 1%-30%; MLNR2, 31%-50%; MLNR3, 51%-100%) were used in our study.
To directly compare the 6th and 7th edition UICC N staging systems with the present MLNR staging system, we took advantage of two statistical methods. One method considers the homogeneity, discriminatory ability and monotonicity of the gradient test. Homogeneity was measured with the likelihood ratio χ2 test related to the Cox regression model. The discriminatory ability and monotonicity of the gradient were measured with the linear trend χ2 test. The likelihood ratio χ2 test was used to assess homogeneity within each classification system and to estimate the gradient monotonicity. Additionally, the Akaike information criterion (AIC) value within a Cox proportional hazard regression model was used to measure the discriminatory ability of each system[24]. The AIC statistic was defined by AIC = -2 log maximum likelihood + 2 × the number of parameters in the model. A smaller AIC value indicates that the model is better at predicting outcome. The other method involves receiver operating characteristic (ROC) curves. ROC curves and the areas under the curves (AUC) were calculated for each of the aforementioned three N staging systems to assess the accuracy of their predictive ability. Differences between the AUC were tested for statistical significance based on the estimated areas and their standard errors[25].
The 5-year survival rate was calculated using the Kaplan-Meier method. The log-rank test was used to make statistical comparisons of different factors. Pearson correlations were examined with a two-tailed test. In multivariate analysis, forward stepwise regression analysis was performed with a Cox proportional hazards model. A P value of ≤ 0.05 was considered statistically significant. All statistical analyses were performed using SPSS software version 16.0 (SPSS Inc., Chicago, IL, United States).
Of the 1042 patients, 708 were male (67.9%) and 334 were female (32.1%). The mean patient age was 57.4 ± 11.5 years (range 20-79 years). The overall 5-year survival rate for all patients was 47.5%, and 474 patients were alive when our follow-up was complete.
After univariate analysis of the 1042 patients who underwent radical resection, ten factors were found to have statistically significant associations with overall survival (OS). They were: age, tumor location, tumor size, histological grade, lymphatic/venous invasion, pT, 6th edition UICC pN, 7th edition UICC pN, MLNR, and the number of retrieved lymph nodes (Table 1). We summarize the postoperative survival results as follows: (1) patients who were older had significantly shorter OS than those who were younger [hazard ratio (HR) = 1.019, P < 0.001]; (2) patients whose primary tumor was located in the distal third of the stomach had significantly longer OS than those whose primary tumor was located elsewhere in the stomach (HR = 0.735, P < 0.001); (3) patients with a larger primary tumor had significantly shorter OS than those with a smaller primary tumor (HR = 1.147, P < 0.001); (4) patients with poorly differentiated or undifferentiated adenocarcinoma had significantly shorter OS than those with well or moderately differentiated adenocarcinoma (HR = 1.254, P < 0.001); (5) patients with tumor lymphatic/venous invasion had significantly shorter OS than those without lymphatic/venous invasion (HR = 2.685, P < 0.001); (6) the deeper the primary tumor invasion, the shorter the OS of the gastric cancer patients (HR = 1.852, P < 0.001); (7) the higher the metastatic lymph node counts of the 6th edition UICC N stage, the shorter the OS of the gastric cancer patients (HR = 1.571, P < 0.001); (8) the higher the metastatic lymph node counts of the 7th edition UICC N stage, the shorter the OS of the gastric cancer patients (HR = 1.604, P < 0.001); (9) the higher the MLNR stage, the shorter the OS of the gastric cancer patients (HR = 1.776, P < 0.001); and (10) patients who had more than 15 lymph nodes retrieved had significantly longer OS than those who had ≤ 15 lymph nodes retrieved (HR = 0.616, P < 0.001). All of the aforementioned 10 variables were included in a multivariate Cox proportional hazards model (forward stepwise procedure) to adjust for the effects of covariates (Table 2). In that model, we demonstrated that age, tumor location, tumor size, histological grade, lymphatic/venous invasion, pT, the 7th edition UICC N staging, MLNR, and the number of retrieved lymph nodes were independent prognostic factors, while the 6th edition UICC N staging was excluded.
| Variable | n (%) | 5-yr survival rate (%) | Log rankχ2value | Hazardratio | P value |
| Gender | 0.433 | 1.060 | 0.511 | ||
| Male | 708 (67.9) | 48.6 | |||
| Female | 334 (32.1) | 45.1 | |||
| Age (continuous) | 1042 (100) | 47.5 | 124.704 | 1.019 | < 0.001 |
| Tumor location | 78.529 | 0.735 | < 0.001 | ||
| Proximal | 579 (55.6) | 39.0 | |||
| Distal | 418 (40.1) | 62.0 | |||
| Two-thirds or more | 45 (4.3) | 17.5 | |||
| Tumor size (continuous) | 47.5 | 124.704 | 1.147 | < 0.001 | |
| Histological grade | 18.407 | 1.254 | < 0.001 | ||
| Well/moderately differentiated adenocarcinoma | 388 (37.2) | 54.8 | |||
| Poorly differentiated adenocarcinoma | 448 (43.0) | 44.7 | |||
| Undifferentiated adenocarcinoma/signet-ring cell carcinoma/mucinous adenocarcinoma | 206 (19.8) | 39.8 | |||
| Lymphatic/venous invasion | 65.905 | 2.685 | < 0.001 | ||
| No | 954 (91.6) | 50.0 | |||
| Yes | 88 (8.4) | 20.4 | |||
| Depth of invasion (7th edition) | 172.456 | 1.852 | < 0.001 | ||
| T1 | 81 (7.8) | 90.7 | |||
| T2 | 120 (11.5) | 74.3 | |||
| T3 | 195 (18.7) | 57.5 | |||
| T4a | 538 (51.6) | 35.9 | |||
| T4b | 108 (10.4) | 22.1 | |||
| The 7th edition UICC N | 168.281 | 1.604 | < 0.001 | ||
| N0 | 332 (31.9) | 71.1 | |||
| N1 | 211 (20.2) | 50.7 | |||
| N2 | 268 (25.7) | 37.5 | |||
| N3 | 231 (22.2) | 22.2 | |||
| The 6th edition UICC N | 160.982 | 1.571 | < 0.001 | ||
| N0 | 332 (31.9) | 71.1 | |||
| N1 | 479 (46.0) | 43.3 | |||
| N2 | 172 (16.5) | 21.4 | |||
| N3 | 59 (5.7) | 25.1 | |||
| Metastatic lymph node ratio | 281.341 | 1.776 | < 0.001 | ||
| MLNR0 | 332 (31.9) | 71.1 | |||
| MLNR1 | 277 (26.6) | 59.0 | |||
| MLNR2 | 154 (14.8) | 32.7 | |||
| MLNR3 | 279 (26.8) | 16.0 | |||
| Retrieved lymph nodes | 67.098 | 0.616 | < 0.001 | ||
| ≤ 15 | 657 (63.1) | 58.0 | |||
| > 15 | 385 (36.9) | 68.4 |
| Variables | Wald | P value | HR | 95% CI |
| Age (continuous) | 23.741 | < 0.001 | 1.020 | 1.012-1.028 |
| Tumor location | 8.825 | 0.003 | 0.794 | 0.682-0.925 |
| Tumor size (continuous) | 29.678 | < 0.001 | 1.085 | 1.054-1.118 |
| Histological grade | 11.542 | 0.001 | 1.222 | 1.089-1.372 |
| Lymphatic/venous invasion | 30.629 | < 0.001 | 2.063 | 1.596-2.666 |
| UICC 7th T | 43.652 | < 0.001 | 1.434 | 1.289-1.596 |
| UICC 7th N | 5.806 | 0.016 | 1.218 | 1.037-1.430 |
| MLNR | 14.693 | < 0.001 | 1.330 | 1.149-1.538 |
| Retrieved lymph nodes | 29.666 | < 0.001 | 0.548 | 0.441-0.680 |
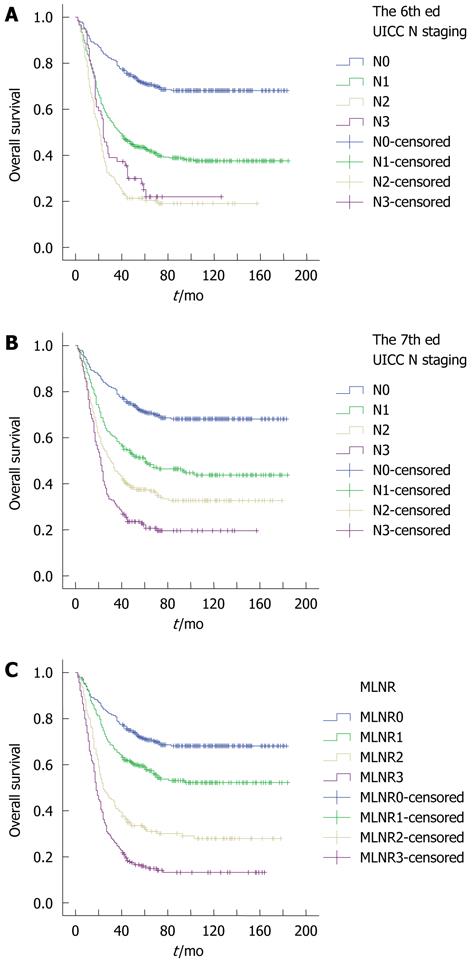
The survival curves developed according to the 6th and 7th edition UICC N staging systems and the MLNR staging system are shown in Figure 1. For all three staging systems, the Kaplan-Meier plot had good discriminatory ability in each group except in N2 and N3 of the 6th edition UICC N staging (Figure 1). The 5-year survival rates of N0, N1, N2, and N3 patients in the 6th edition UICC N staging were 71.1%, 43.3%, 21.4%, and 25.1%, respectively (P < 0.001, P < 0.001 and P = 0.143, respectively). The 5-year survival rates of N0, N1, N2, and N3 patients in the 7th edition UICC N staging were 71.1%, 50.7%, 37.5%, and 22.2%, respectively (P < 0.001, P = 0.003 and P = 0.001, respectively). The 5-year survival rates of MLNR0, MLNR1, MLNR2, and MLNR3 patients were 71.1%, 59.0%, 32.7% and 16.0%, respectively (P < 0.001, P < 0.001 and P < 0.001, respectively).
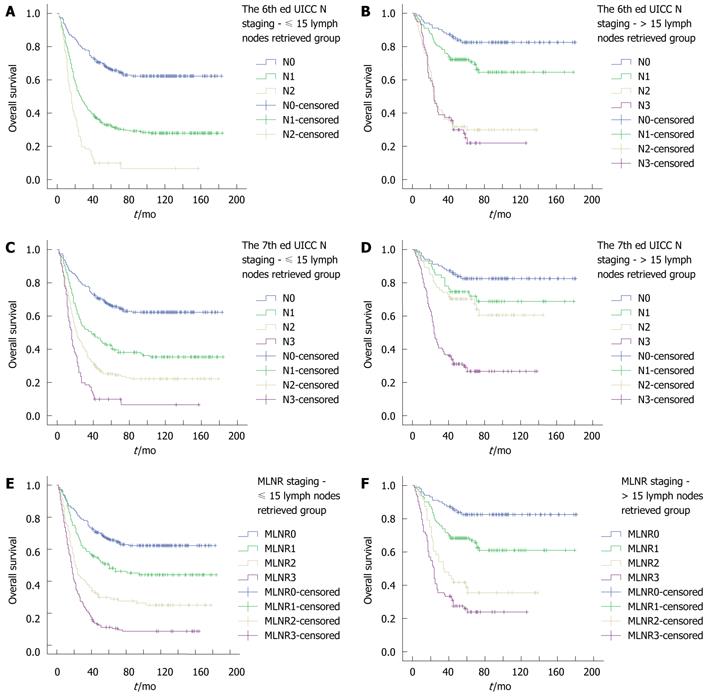
We also investigated the impact of the number of lymph nodes retrieved on OS rates according to different N staging systems. In the 6th edition UICC N staging, the 5-year survival rate was significantly higher for patients with N0 compared with N1 and N2 in the ≤ 15 lymph nodes retrieved group, in which no patient was classified as N3 (P < 0.001 and P < 0.001, respectively). The Kaplan-Meier plot discriminated well between each N staging in the > 15 lymph nodes retrieved group (P = 0.008 and P < 0.001, respectively), except for N2 vs N3 (P = 0.720). As for the 7th edition UICC N staging, the Kaplan-Meier plot discriminated well between each N staging in both the ≤ 15 and > 15 lymph nodes retrieved groups, except for N0 vs N1 and N1 vs N2 (P = 0.070 and P = 0.433, respectively) in the > 15 lymph nodes retrieved group. When we investigated the MLNR in the ≤ 15 and > 15 lymph nodes retrieved groups, the Kaplan-Meier plot showed that the 5-year survival rate was significantly different for each MLNR stage. In the ≤ 15 lymph nodes retrieved group, the 5-year OS was 66.2%, 49.9%, 29.9%, and 11.2% for MLNR 0, 1, 2, and 3 (P = 0.001, P < 0.001 and P < 0.001, respectively). In the > 15 lymph nodes retrieved group, the 5-year OS was 82.5%, 68.3%, 38.7%, and 25.8% for MLNR 0, 1, 2, and 3 (P = 0.001, P < 0.001 and P = 0.038, respectively) (Figure 2).
The performance of the 6th and 7th edition UICC N staging systems and the MLNR staging, as assessed by the linear trend χ2, likelihood ratio χ2, and the AIC test, is described in Table 3. Compared with the 6th and 7th edition UICC N staging systems, the MLNR staging had better homogeneity (higher likelihood ratio χ2 score), discriminatory ability, and monotonicity of gradients (higher linear trend χ2 score). Furthermore, the MLNR staging had a smaller AIC value, representing the optimum prognostic stratification.
| Classification | Subgroups | Linear trendχ2 | Likelihood ratioχ2 | AIC |
| 6th ed UICC N staging | N 0, 1, 2, 3 | 117.751 | 141.517 | 7364.073 |
| 7th ed UICC N staging | N 0, 1, 2, 3 | 138.342 | 146.796 | 7325.731 |
| MLNR staging | MLNR 0, 1, 2, 3 | 203.476 | 219.912 | 7240.017 |
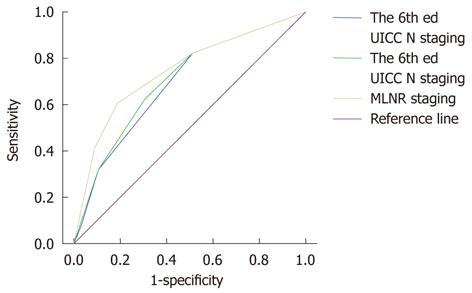
Finally, we used the ROC curves of the three aforementioned N staging systems to calculate the AUC and thus to assess the accuracy of each system’s predictive ability for gastric cancer patients who underwent radical resection (Figure 3). The AUC was 0.692 for the 6th edition UICC N staging, 0.705 for the 7th edition UICC N staging, and 0.754 for the MLNR staging, indicating that the MLNR staging was superior to the 6th and 7th edition UICC N staging systems and could be used as a more precise prognostic staging tool for gastric cancer patients.
Because the 6th edition UICC TNM staging system is simple, reliable, and reproducible, it is currently used all over the world. For cases in which < 15 lymph nodes are examined, N stage may be incorrect because of stage migration. A method for bypassing this problem is to consider the ratio between metastatic and examined lymph nodes. Studies have demonstrated that staging by the MLNR is superior to staging by the absolute number of metastatic lymph nodes (such as in the 6th edition UICC N staging) for predicting prognosis of gastric cancer patients[14,26,27]. Furthermore, some reports have demonstrated that the 7th edition UICC N staging is more suitable for prognosis than the 6th edition system[18,28]. For example, although all the patients in our study underwent D2 gastrectomy with R0 resection, the number of lymph nodes recovered in the majority of patients (63.1%) was no more than 15, and therefore, in these patients, the N stage cannot be classified as N3 according to the 6th edition UICC staging system. On the other hand, in the 7th edition system, patients may be classified as N3 as long as the number of retrieved lymph nodes is more than 7, and thus, this revised edition system may reduce stage migration. Whether the 7th edition UICC N staging is optimal is still unknown. To our knowledge, although a few documents claim that the 7th edition UICC N staging is superior to the 6th edition system, there are no formal studies that have examined the superiority of the MLNR to the 7th edition UICC N staging to date.
In this study, the MLNR was one of the most important prognostic factors of gastric cancer mortality. The MLNR provided a better classification of patient prognostic risk profiles than the 6th and 7th edition UICC N classification systems, particularly in the analysis stratified by the number of lymph nodes retrieved. The MLNR shows a clear advantage over the 6th and 7th edition UICC N staging systems for the following reasons: first, in univariate analysis, the log-rank χ2 associated with the MLNR (χ2 = 281.341) was larger than that of the 6th and 7th edition UICC N staging systems (χ2 = 160.982 and χ2 = 168.281, respectively), indicating a higher statistical significance (Table 1); second, in multivariate analysis, the HR was higher in the MLNR (HR = 1.330, 95% CI: 1.149-1.538) staging than in the 7th edition UICC N staging (HR = 1.218, 95% CI: 1.037-1.430) (Table 2); third, although the 6th and 7th edition UICC N classifications discriminated well between each group, the MLNR provided a better classification of patient prognostic risk profiles than the pN stage, particularly in the analysis stratified by the number of lymph nodes retrieved (Figure 2); fourth, the MLNR staging had better homogeneity (higher likelihood ratio χ2 score, 219.912 vs 146.796 vs 141.517), discriminatory ability, and monotonicity of the gradients (higher linear trend χ2 score, 203.476 vs 138.342 vs 117.751) and a smaller AIC value (7240.017 vs 7325.731 vs 7364.073) (Table 3); and finally, the AUC under the ROC curve was larger in the MLNR staging (0.754) than in the 6th and 7th edition UICC N staging systems (0.692 and 0.705, respectively), indicating that MLNR staging was superior to the 6th and 7th edition UICC N staging methods and could be used as a more precise prognostic staging tool for gastric cancer patients.
According to Ueno et al[29], the performance of a staging system can be evaluated as follows: homogeneity within subgroups (small differences in survival among patients with the same stage), discriminatory ability between different groups (large differences in survival among patients in different stages), and monotonicity of the gradients shown in the correlation between stages and survival rates (within the same system, patients in earlier stages have longer survival than those in later stages). In our study, the MLNR staging had better homogeneity (higher likelihood ratio χ2 score), discriminatory ability, and monotonicity of the gradients (higher linear trend χ2 score) than did the 6th and 7th edition UICC N staging systems. Furthermore, in our study, the MLNR staging had a smaller AIC value, indicating that the MLNR staging has the optimum prognostic stratification and smallest loss of information for predicting outcome[30,31]. Additionally, the AUC under the ROC curve was larger in the MLNR staging than the aforementioned two N staging systems. These results demonstrate that the MLNR staging has better prognostic stratification and more precise prediction than do the 6th and 7th edition TNM staging systems.
Although the body of literature regarding the MLNR is growing, many studies have been performed using diverse patient groups and different surgical techniques. The cut-points for the MLNR have not necessarily been discussed adequately or validated in alternative data sets. We believe that systematic MLNR analyses of multi-institutional, randomized patient data with validation in similar independent data sets are required to clearly demonstrate the importance of the MLNR. Although the current UICC TNM staging system is the most basic and prevalent for predicting the survival of gastric cancer patients with radical resection, we believe that it will be essential to consider a staging system that includes accurate prognostic variables such as the MLNR in the near future. For all these reasons, the potential advantages of incorporating the MLNR in staging systems should be investigated in large, prospective data sets.
In conclusion, our study compared three lymph node based N staging systems for gastric cancer patients who underwent radical resection and D2 lymphadenectomy and then demonstrated that the MLNR categories could define gastric cancer prognosis more adequately and precisely than the 6th and 7th edition UICC N categories. We propose that nodal ratios should be considered as an alternative to the current UICC N staging.
In recent years, more new cases of gastric cancer are diagnosed in China each year than in any other country. The most powerful and reliable factors which have been widely used are the tumor-node-metastasis classification proposed by the International Union Against Cancer (UICC).
Although UICC N staging is a convenient and reproducible method for precise staging, it demands the examination of at least 15 lymph nodes. If the number of dissected and examined lymph nodes is small or large, downmigration or upmigration of N stage may occur. The metastatic lymph node ratio (MLNR), defined as the number of positive lymph nodes divided by the number of lymph nodes retrieved, has been considered as an alternative to the absolute number of positive lymph nodes.
In the year 2010, staging of lymph node metastasis in gastric cancer has changed in both the UICC 7th edition staging system and in the Japanese Gastric Cancer Association 14th edition system to depend solely on the number of metastatic nodes found. Some authors have demonstrated that the 7th edition UICC staging system was superior to the 6th edition in aspects of homogeneity and discriminatory ability with prognostic value. However, there has been no formal proposal to date focused on comparing the prognostic significance between the MLNR and the 7th edition UICC N staging systems. In the present article, we investigated whether patients with gastric cancer can be classified into meaningful risk categories based on LNR, by comparing this staging with the 7th edition UICC N staging.
This study compared three lymph node based N staging systems for gastric cancer patients with radical resection and D2 lymphadenectomy, and then demonstrated that the MLNR categories could define gastric cancer prognosis more adequately and precisely than the 6th and 7th edition UICC N categories. The authors suggest that nodal ratios should be considered as a countermeasure to the current UICC N staging.
This is a large study of 1042 gastric cancer patients undergoing radical resection plus D2 lymphadenectomy, with a mean follow-up of 56 mo. The authors have analyzed patient outcomes in considerable depth, their data is well characterized. They provide an in depth analysis of factors contributing to survival and have utilized multivariate analysis in doing this. The information in the manuscript is highly relevant and useful.
Peer reviewer: Lygia Stewart, MD, Professor of Clinical Surgery, University of California San Francisco, 4150 Clement Street, San Francisco, CA 94121, United States
S- Editor Lv S L- Editor Webster JR E- Editor Zheng XM
| 1. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7953] [Cited by in F6Publishing: 8028] [Article Influence: 535.2] [Reference Citation Analysis (2)] |
| 2. | Sobin LH, Wittekind C. TNM classification of malignant tumours. 6th ed. Hoboken, NJ: John Wiley & Sons 2002; . [Cited in This Article: ] |
| 3. | Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumors. 7th ed. Oxford: Wiley-Blackwell 2009; . [Cited in This Article: ] |
| 4. | Yokota T, Ishiyama S, Saito T, Teshima S, Narushima Y, Murata K, Iwamoto K, Yashima R, Yamauchi H, Kikuchi S. Lymph node metastasis as a significant prognostic factor in gastric cancer: a multiple logistic regression analysis. Scand J Gastroenterol. 2004;39:380-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 102] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Yoo CH, Noh SH, Kim YI, Min JS. Comparison of prognostic significance of nodal staging between old (4th edition) and new (5th edition) UICC TNM classification for gastric carcinoma. International Union Against Cancer. World J Surg. 1999;23:492-47; discussion 492-47;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Hermanek P. pTNM and residual tumor classifications: problems of assessment and prognostic significance. World J Surg. 1995;19:184-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 137] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med. 1985;312:1604-1608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1124] [Cited by in F6Publishing: 1108] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 8. | Ichikawa D, Kurioka H, Ueshima Y, Shirono K, Kan K, Shioaki Y, Lee CJ, Hamashima T, Deguchi E, Ikeda E. Prognostic value of lymph node staging in gastric cancer. Hepatogastroenterology. 2003;50:301-304. [PubMed] [Cited in This Article: ] |
| 9. | Coburn NG, Swallow CJ, Kiss A, Law C. Significant regional variation in adequacy of lymph node assessment and survival in gastric cancer. Cancer. 2006;107:2143-2151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 153] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 10. | Aurello P, D'Angelo F, Rossi S, Bellagamba R, Cicchini C, Nigri G, Ercolani G, De Angelis R, Ramacciato G. Classification of lymph node metastases from gastric cancer: comparison between N-site and N-number systems. Our experience and review of the literature. Am Surg. 2007;73:359-366. [PubMed] [Cited in This Article: ] |
| 11. | Kulig J, Sierzega M, Kolodziejczyk P, Popiela T. Ratio of metastatic to resected lymph nodes for prediction of survival in patients with inadequately staged gastric cancer. Br J Surg. 2009;96:910-918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Sun Z, Zhu GL, Lu C, Guo PT, Huang BJ, Li K, Xu Y, Li DM, Wang ZN, Xu HM. The impact of N-ratio in minimizing stage migration phenomenon in gastric cancer patients with insufficient number or level of lymph node retrieved: results from a Chinese mono-institutional study in 2159 patients. Ann Oncol. 2009;20:897-905. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | Marchet A, Mocellin S, Ambrosi A, Morgagni P, Garcea D, Marrelli D, Roviello F, de Manzoni G, Minicozzi A, Natalini G. The ratio between metastatic and examined lymph nodes (N ratio) is an independent prognostic factor in gastric cancer regardless of the type of lymphadenectomy: results from an Italian multicentric study in 1853 patients. Ann Surg. 2007;245:543-552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 269] [Cited by in F6Publishing: 300] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 14. | Kim CY, Yang DH. Adjustment of N stages of gastric cancer by the ratio between the metastatic and examined lymph nodes. Ann Surg Oncol. 2009;16:1868-1874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Inoue K, Nakane Y, Iiyama H, Sato M, Kanbara T, Nakai K, Okumura S, Yamamichi K, Hioki K. The superiority of ratio-based lymph node staging in gastric carcinoma. Ann Surg Oncol. 2002;9:27-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 188] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 16. | Nitti D, Marchet A, Olivieri M, Ambrosi A, Mencarelli R, Belluco C, Lise M. Ratio between metastatic and examined lymph nodes is an independent prognostic factor after D2 resection for gastric cancer: analysis of a large European monoinstitutional experience. Ann Surg Oncol. 2003;10:1077-1085. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 154] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 17. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma. 14th ed. Tokyo: Kanehara & Co. Ltd 2010; . [Cited in This Article: ] |
| 18. | Wang W, Sun XW, Li CF, Lv L, Li YF, Chen YB, Xu DZ, Kesari R, Huang CY, Li W. Comparison of the 6th and 7th editions of the UICC TNM staging system for gastric cancer: results of a Chinese single-institution study of 1,503 patients. Ann Surg Oncol. 2011;18:1060-1067. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Rausei S, Dionigi G, Boni L, Rovera F, Dionigi R. How does the 7th TNM edition fit in gastric cancer management? Ann Surg Oncol. 2011;18:1219-1221. [PubMed] [Cited in This Article: ] |
| 20. | Deng J, Liang H, Wang D. The feasibility of N stage of the 7th edition TNM for gastric cancer. Ann Surg Oncol. 2011;18:1805-1806. [PubMed] [Cited in This Article: ] |
| 21. | Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition. Gastric Cancer. 1998;1:10-24. [PubMed] [Cited in This Article: ] |
| 22. | Celen O, Yildirim E, Berberoglu U. Prognostic impact of positive lymph node ratio in gastric carcinoma. J Surg Oncol. 2007;96:95-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Saito H, Fukumoto Y, Osaki T, Yamada Y, Fukuda K, Tatebe S, Tsujitani S, Ikeguchi M. Prognostic significance of the ratio between metastatic and dissected lymph nodes (n ratio) in patients with advanced gastric cancer. J Surg Oncol. 2008;97:132-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Harrell FE, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982;247:2543-2546. [PubMed] [Cited in This Article: ] |
| 25. | Yu JW, Wu JG, Zheng LH, Zhang B, Ni XC, Li XQ, Jiang BJ. Influencing factors and clinical significance of the metastatic lymph nodes ratio in gastric adenocarcinoma. J Exp Clin Cancer Res. 2009;28:55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Xu DZ, Geng QR, Long ZJ, Zhan YQ, Li W, Zhou ZW, Chen YB, Sun XW, Chen G, Liu Q. Positive lymph node ratio is an independent prognostic factor in gastric cancer after d2 resection regardless of the examined number of lymph nodes. Ann Surg Oncol. 2009;16:319-326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 27. | Maduekwe UN, Lauwers GY, Fernandez-Del-Castillo C, Berger DL, Ferguson CM, Rattner DW, Yoon SS. New metastatic lymph node ratio system reduces stage migration in patients undergoing D1 lymphadenectomy for gastric adenocarcinoma. Ann Surg Oncol. 2010;17:1267-1277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Deng J, Liang H, Sun D, Wang D, Pan Y. Suitability of 7th UICC N stage for predicting the overall survival of gastric cancer patients after curative resection in China. Ann Surg Oncol. 2010;17:1259-1266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 29. | Ueno S, Tanabe G, Sako K, Hiwaki T, Hokotate H, Fukukura Y, Baba Y, Imamura Y, Aikou T. Discrimination value of the new western prognostic system (CLIP score) for hepatocellular carcinoma in 662 Japanese patients. Cancer of the Liver Italian Program. Hepatology. 2001;34:529-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 228] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 30. | Kee KM, Wang JH, Lee CM, Chen CL, Changchien CS, Hu TH, Cheng YF, Hsu HC, Wang CC, Chen TY. Validation of clinical AJCC/UICC TNM staging system for hepatocellular carcinoma: analysis of 5,613 cases from a medical center in southern Taiwan. Int J Cancer. 2007;120:2650-2655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Cho YK, Chung JW, Kim JK, Ahn YS, Kim MY, Park YO, Kim WT, Byun JH. Comparison of 7 staging systems for patients with hepatocellular carcinoma undergoing transarterial chemoembolization. Cancer. 2008;112:352-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |