Published online Nov 14, 2011. doi: 10.3748/wjg.v17.i42.4675
Revised: June 10, 2011
Accepted: June 17, 2011
Published online: November 14, 2011
AIM: To determine the apoptosis pathway in residual viable hepatocellular carcinoma (HCC) tissues following transarterial embolization (TAE).
METHODS: Ten patients with HCC who received surgical resection after TAE were enrolled in the study group, and 24 patients with HCC who received surgical resection only served as the control group. In the study group, we measured the changes in tumor size and α fetoprotein (AFP) levels after TAE. All tissue samples were taken from the residual tumors. The expression of various apoptotic proteins was evaluated via immunoblotting procedures. The results were analyzed using a Student’s t test.
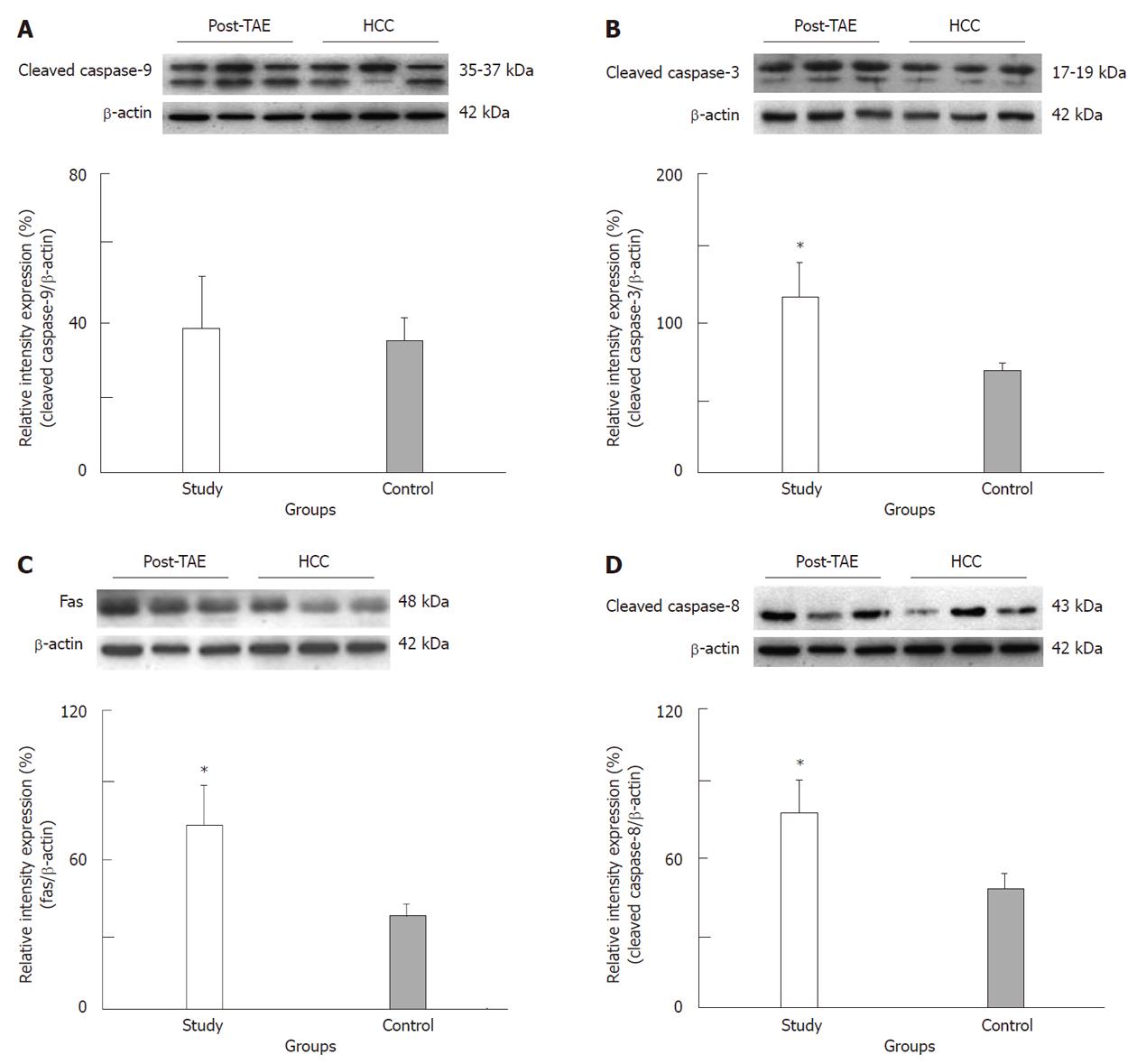
RESULTS: Tumor size and the AFP level decreased by 46.2% and 55.3% after TAE, respectively. There was no significant difference detected for the Bcl-2/Bax ratio or the cleaved caspase-9 expression levels in either group. However, extrinsic apoptopic pathway-related expression of Fas and cleaved caspase-8 expression were significantly higher in the study group than in the control group (P < 0.05). In addition, cleaved caspase-3 expression in the study group was higher (1.62-fold) than in control group (P < 0.05).
CONCLUSION: TAE is an effective palliative therapy that decreases tumor size and AFP levels via an increase in extrinsic apoptosis pathway in patients with unresectable HCC.
- Citation: Wang SH, Chen LM, Yang WK, Lee JD. Increased extrinsic apoptotic pathway activity in patients with hepatocellular carcinoma following transarterial embolization. World J Gastroenterol 2011; 17(42): 4675-4681
- URL: https://www.wjgnet.com/1007-9327/full/v17/i42/4675.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i42.4675
Hepatocellular carcinoma (HCC) is one of the most common malignant cancers in the world[1,2]. Generally, HCC is induced by chronic liver injury such as liver cirrhosis. The chronic injury leads to a change in the cellular properties of the liver and curtails the delivery of blood throughout the liver. Subsequently, this pathological change leads to a reduction in blood circulation throughout the liver and accompanying hypoxia. The increased cellularity associated with the highly proliferative tumor cells also induces local hypoxia inside the actual HCC[3]. Hypoxia can stimulate angiogenesis to support tumor growth by inducing angiogenic factors[3,4]. Many people with advanced HCC have tumors that are unresectable by the time of disease diagnosis. Transarterial embolization (TAE) appears to be as effective as transarterial chemoembolization (TACE) and plays a major role in the treatment of patients with advanced HCC[5,6]. However, the exact molecular changes and apoptotic pathway activity of cancerous tissues following embolization still needs to be elucidated.
Several studies have reported that TACE inhibits tumor angiogenesis and induces tumor cell apoptosis. Other studies have found that TACE stimulates tumor angiogenesis and increases the proliferative activity of the tumor cells due to anoxic stress-related Bcl-2 overexpression[7,8]. Unfortunately, most previous papers neglected to examine the counter factor of apoptotic protein (Bax) expression in residual viable cancerous tissues following TAE in HCC patients. We examined the expression of apoptotic proteins in the specimens taken from both groups (surgical resection only and surgery after TAE) of HCC patients. To determine whether the intrinsic or extrinsic apoptotic pathways were involved, we examined the Bcl-2/Bax ratio (anti-apoptosis protein/an inducer of apoptosis), cleaved caspase-9, Fas, cleaved caspase-8 and cleaved caspase-3 expression levels.
To our knowledge, this is the first report to study the apoptotic pathway in the residual viable cancerous tissues of HCC patients following TAE. A better understanding of the molecular changes and apoptotic pathways associated with HCC will aid in the development of more effective future therapies.
From January 2006 to December 2009, we enrolled 10 patients (6 men and 4 women; average age, 53.6 years) with HCC as a study group. All 10 patients received TAE with 95% alcohol and Gelform due to poor liver function and relative high initial risks for operation. The study group patients then underwent hepatic resection three weeks after the TAE. During the same period, we enrolled 24 patients (16 men and 8 women; average age, 54.4 years) who only received surgical resection for HCC as a control group. All liver tumor tissues were stored at -80 °C for immunoblotting (including Bcl-2/Bax ratio, cleaved caspase-9, Fas, cleaved caspase-8 and cleaved caspase-3) or fixed in formalin for immunohistochemical (IHC) staining (including caspase-9, caspase-8 and cleaved caspase-3). Simultaneously, we measured the changes in tumor size and α fetoprotein (AFP) in the study group after TAE. The specimens were removed only after written informed consent was obtained from the patients. This study was approved by the Institute Ethics Committee of Taichung Armed Forces General Hospital.
Nine types of primary antibodies were used in the present study: (1) Bcl-2, a mouse monoclonal antibody (sc-7382, Santa Cruz, Santa Cruz, CA, United States; dilution 1:500 for immunoblotting); (2) Bax, a mouse monoclonal antibody (sc-7480, Santa Cruz; dilution 1:200 for immunoblotting); (3,4) caspase-9, two rabbit polyclonal antibodies for immunoblotting (No. 9502, Cell Signaling, Beverly, MA, United States; dilution 1:1000) and IHC staining (sc-8355, Santa Cruz; dilution 1:100); (5) Fas, a rabbit polyclonal antibody (sc-7886, Santa Cruz; dilution 1:400 for immunoblotting); (6,7) caspase-8, a mouse monoclonal antibody for immunoblotting (No. 9746, Cell Signaling; dilution 1:1500) and a goat polyclonal antibody (sc-6134, Santa Cruz; dilution 1:50 for IHC staining); (8) cleaved caspase-3, a rabbit monoclonal antibody (No. 9664, Cell Signaling; dilution 1:1000 and 1:50 for immunoblotting and IHC staining, respectively); and (9) β-actin, a mouse monoclonal antibody (No. 8226, Abcam, Cambridge, MA, United States; dilution 1:8000 for immunoblotting). The secondary antibodies used were horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (No. 0031430, Pierce, Hercules, CA, United States; for detection of Bcl-2, Bax, caspase-8 and β-actin) and goat anti-rabbit IgG (No. 0031460, Pierce; for detection of caspase-9, Fas and cleaved caspase-3) for immunoblotting or a commercial kit (PicTureTM; Zymed, South San Francisco, CA, United States) for IHC staining.
The immunoblotting method was modified from our previous studies[9,10]. In brief, aliquots containing 100 μg of sample supernatants were added to the sample buffer and heated at 95 °C for 5 min. After electrophoresis and transformation, blots (polyvinylidene fluoride membranes; Millipore, Bedford, MA, United States) were preincubated in phosphate buffer saline with 0.05% Tween 20 (PBST) buffer containing 5% (wt/vol) nonfat dried milk to minimize non-specific binding. According to the manufacturer’s manual, Bcl-2, Bax, cleaved caspase-9, Fas, cleaved caspase-8, cleaved caspase-3 and β-actin antibodies should bind to proteins with molecular weights of about 26, 23, 35-37, 48, 43, 17-19 and 44 kDa, respectively. The blots were cut into upper and lower parts at feasible sites for the incubations. The blots were incubated with the primary antibodies diluted in 1% bovine serum albumin and 0.05% sodium azide in PBST. The blots were then incubated with HRP-conjugated secondary antibody diluted in PBST. The blots were developed using a SuperSignal West Pico Detection Kit (No. 34082, Pierce). After development, the immunoblots were scanned and imported as TIFF files. All immunoreactive bands were analyzed using MCID software version 7.0 (Imaging Research, Ontario, Canada). The results were converted to numerical values to compare the relative protein abundance of the immunoreactive bands.
The immunohistochemical (IHC) staining method used was modified from our previous study[9]. In brief, the sections were stained with primary antibody (caspase-9, caspase-8 or cleaved caspase-3) or PBS (negative control) before being analyzed with the commercial kit. The sections were observed under light microscope (model BX50; Olympus, Tokyo, Japan), and the micrographs were reviewed.
All data were analyzed using the Student’s t test with P < 0.05 being considered statistically significantly different. Values were expressed as the mean ± SE.
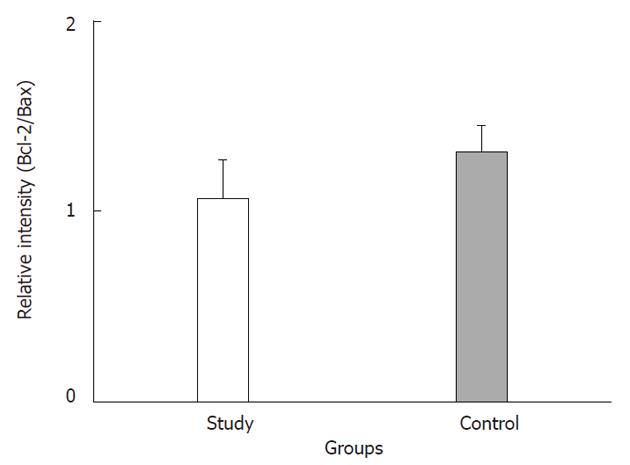
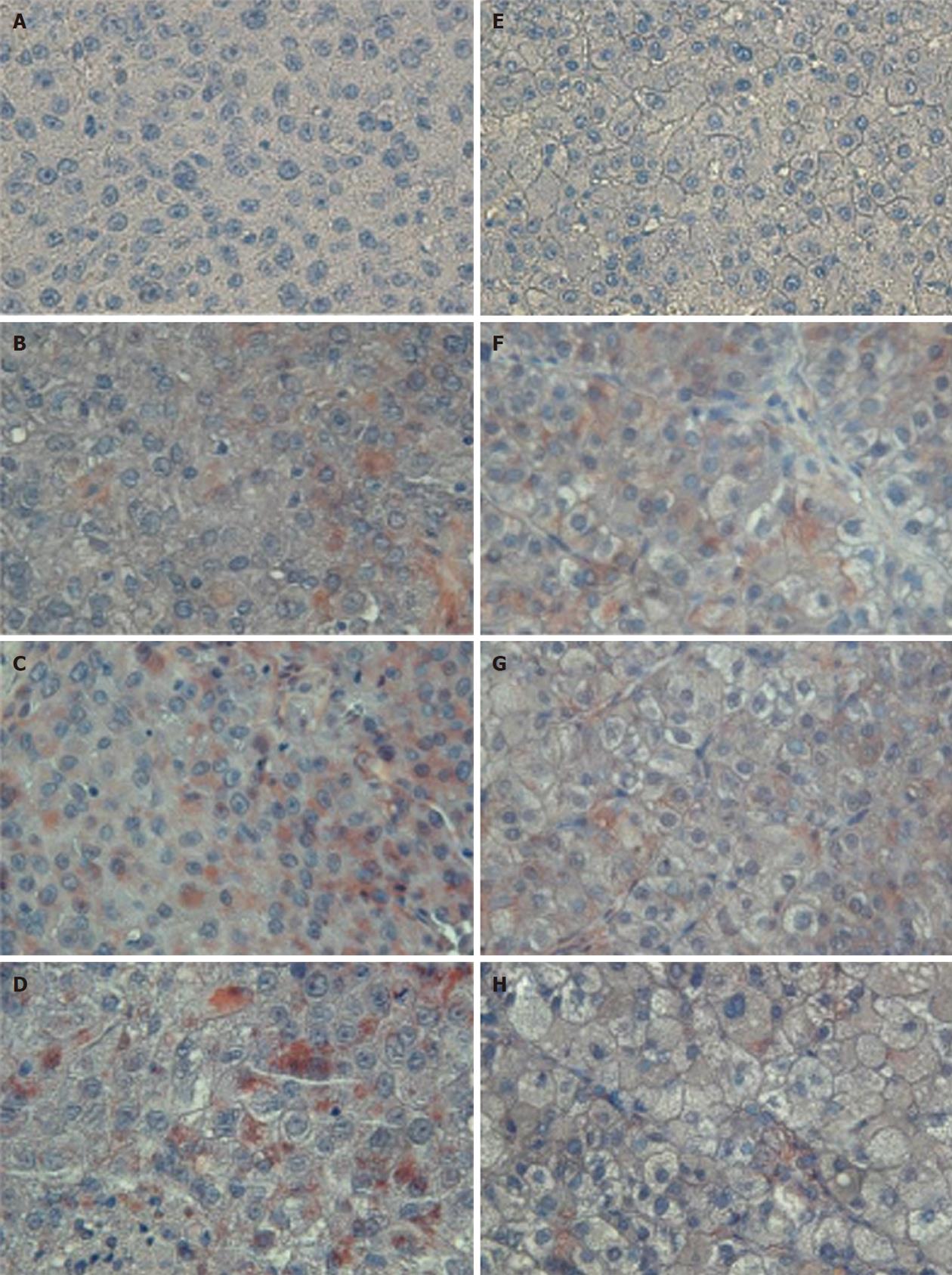
The tumor size and AFP levels decreased by about 46.2% and 55.3%, respectively, following TAE (Table 1). The Bcl-2/Bax ratio and cleaved caspase-9 expression levels, both indicative of the intrinsic apoptosis pathway, demonstrated no statistical differences between the groups (P > 0.05; Figures 1 and 2A; Table 2). However, the expression levels of Fas and cleaved caspase-8, both involved in the extrinsic apoptosis pathway, were significantly higher in the study group (P < 0.05; Figure 2C and D; Table 2). Cleaved caspase-3 expression in the study group was also higher than in the control group (P < 0.05; Figure 2B; Table 2). Representative sections from study group patients (Figure 3A-D) and control group patients (Figure 3E-H) for IHC staining (caspase-9, caspase-8 and cleaved caspase-3) supported the immunoblotting results.
| Items | Pre-TAE | Post-TAE | Decreased ratio (%)1 |
| Tumor size (cm3) | |||
| Mean | 70.15 | 34.2 | 46.2 |
| Range | 10.4-172.3 | 6.0-67.5 | 33.0-60.8 |
| AFP (ng/mL) | |||
| Mean | 120.5 | 53.7 | 55.3 |
| Range | 11.7-319.0 | 3.6-159.7 | 8.5-92.3 |
These findings demonstrated that the decreased tumor size and AFP levels in HCC patients following TAE resulted from increased activity of the extrinsic apoptosis pathway.
HCC is one of the most common and lethal malignant tumors worldwide. The late diagnosis and advanced underlying liver cirrhosis responsible for HCC generally makes complete resection non-viable. TACE is one of the most validated treatments for unresectable HCC in patients with preserved liver function[11-13]. Several studies have reported that TACE inhibits tumor angiogenesis and induces tumor cell apoptosis[7,14]. Conversely, other studies indicate that TACE stimulates tumor angiogenesis and increases proliferative activity of the tumor cells due to hypoxic stress-induced Bcl-2 overexpression[7,8]. Hypoxia enhances proliferation, angiogenesis, metastasis, chemoresistance and radioresistance to suppress differentiation and apoptosis of HCC[15]. Therefore, combining therapies that induce and target hypoxia may be beneficial to HCC patients. TAE appears to be as effective as TACE in improving survival rates of cirrhotic HCC patients[5,6,16]. TAE allows local tumor response to reach 88%, and in addition, bleeding control can be increased from 83% to 100%[17,18]. However, there have been no further studies investigating the molecular changes and apoptotic pathways of residual viable HCC tissues following TAE.
Apoptosis, in multicellular organisms, plays an important role in development and tissue homeostasis[19-21]. The two major mechanisms of cell death are referred to as the intrinsic and extrinsic pathways. The intrinsic (or mitochondrial) pathway is induced by cellular stress, which involves Bcl-2, Bax, mitochondrial outer-membrane permeability and caspase-9 protein. The extrinsic (or death receptor) pathway is induced by specific ligands that engage death receptors, which generally involves Fas and the binding and activation of caspase-8 protein[22-24]. In the human apoptotic pathway cascade, 14 caspases (cysteinyl aspartate-specific proteinases) have been discovered to date[25,26]. Among them, caspase-3 is considered the most crucial executioner protease, as it is essential for apoptotic death in mammalian cells[27].
In this study, tumor size and AFP level were decreased by 46.2% and 55.3% following TAE, respectively. There was no significant difference of Bcl-2/Bax ratio and cleaved caspase-9 expression in either group. However, the expression levels of Fas and cleaved caspase-8, both involved in the extrinsic pathway, were significantly higher in the study group than in control group. In addition, increased cleaved caspase-3 expression was also observed in the TAE group (1.62-fold over the control group level). These findings demonstrate increased apoptosis through the extrinsic pathway in HCC patients following TAE. Previous studies had not examined the expression of proliferative and apoptotic proteins (Bcl-2 and Bax) in residual viable HCC tissues simultaneously.
In conclusion, our data indicate that TAE is an effective palliative therapy that decreases tumor size and AFP levels via increasing the extrinsic apoptosis pathway in patients with unresectable HCC. Thus, it would be of interest to investigate whether increasing the expression of extrinsic apoptotic proteins could provide more effective treatment for patients with HCC.
Transarterial embolization (TAE) plays a major role in the treatment of patients with unresectable hepatocellular carcinoma (HCC). However, the apoptotic pathway of residual viable HCC tissues following TAE remains unclear. Elucidating the apoptosis pathway associated with residual viable HCC following a TAE procedure should aid in the development of more effective therapies in the future.
To evaluate potential therapeutic effects, the authors examined the changes in tumor size and α fetoprotein (AFP) levels following TAE. There are two major mechanisms of cell death, and the intrinsic and extrinsic pathways. The intrinsic (or mitochondrial) pathway is induced by cellular stress, which involves Bcl-2, Bax and caspase-9 protein. The extrinsic (or death receptor) pathway is induced by specific ligands that engage death receptors, which involves Fas and the binding and activation of caspase-8 protein. Caspase-3 is considered as the crucial executioner protease because it is essential for apoptotic death in mammalian cells. All of these apoptotic proteins were examined during this study.
This is the first report to study the apoptotic pathway in the residual viable cancerous tissues of patients with HCC following TAE. A better understanding of the molecular changes in and apoptotic pathways of HCC should lead to the development of more efficacious therapies in the future.
TAE is an effective therapy for decreasing tumor size and AFP levels via increasing the extrinsic pathway apoptosis in patients with unresectable HCC. Thus, it would be of interest to investigate whether further increasing the expression of extrinsic apoptotic proteins could provide more effective treatment for patients with HCC.
This is a study showing different expression of apoptotic proteins in residual viable HCC tissues in patients subjected to TAE or not. The authors demonstrated that in TAE group there is a significant increase of apoptosis through extrinsic pathway.
Peer reviewer: Samir Ahboucha, PhD, Assistant Professor, Équipe Neurosciences, Pharmacologie et Environnement, Université Cadi Ayyad, Faculté des Sciences Semlalia, Avenue My Abdellah, BP 2390, Marrakech 40000, Morocco
S- Editor Tian L L- Editor Ma JY E- Editor Zhang DN
| 1. | McKillop IH, Moran DM, Jin X, Koniaris LG. Molecular pathogenesis of hepatocellular carcinoma. J Surg Res. 2006;136:125-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Kusakabe A, Tanaka Y, Orito E, Sugauchi F, Kurbanov F, Sakamoto T, Shinkai N, Hirashima N, Hasegawa I, Ohno T. A weak association between occult HBV infection and non-B non-C hepatocellular carcinoma in Japan. J Gastroenterol. 2007;42:298-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Kim KR, Moon HE, Kim KW. Hypoxia-induced angiogenesis in human hepatocellular carcinoma. J Mol Med (Berl). 2002;80:703-714. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Gwak GY, Yoon JH, Kim KM, Lee HS, Chung JW, Gores GJ. Hypoxia stimulates proliferation of human hepatoma cells through the induction of hexokinase II expression. J Hepatol. 2005;42:358-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 133] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Marelli L, Stigliano R, Triantos C, Senzolo M, Cholongitas E, Davies N, Tibballs J, Meyer T, Patch DW, Burroughs AK. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol. 2007;30:6-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 583] [Cited by in F6Publishing: 590] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 6. | Pleguezuelo M, Marelli L, Misseri M, Germani G, Calvaruso V, Xiruochakis E, Pinelopi M, Burroughs AK. TACE versus TAE as therapy for hepatocellular carcinoma. Expert Rev Anticancer Ther. 2008;8:1623-1641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ, Cao GW. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008;49:523-529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 210] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 8. | Kobayashi N, Ishii M, Ueno Y, Kisara N, Chida N, Iwasaki T, Toyota T. Co-expression of Bcl-2 protein and vascular endothelial growth factor in hepatocellular carcinomas treated by chemoembolization. Liver. 1999;19:25-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 57] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Lee JD, Lee TH, Cheng WH, Jeng SY. Involved intrinsic apoptotic pathway of testicular tissues in varicocele-induced rats. World J Urol. 2009;27:527-532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Lee JD, Yang WK, Lai CH. Involved intrinsic apoptotic pathway in the varicocele and varicose veins. Ann Vasc Surg. 2010;24:768-774. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Maleux G, van Malenstein H, Vandecaveye V, Heye S, Vaninbroukx J, Nevens F, Verslype C. Transcatheter chemoembolization of unresectable hepatocellular carcinoma: current knowledge and future directions. Dig Dis. 2009;27:157-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Tsochatzis EA, Germani G, Burroughs AK. Transarterial chemoembolization, transarterial chemotherapy, and intra-arterial chemotherapy for hepatocellular carcinoma treatment. Semin Oncol. 2010;37:89-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Liapi E, Geschwind JF. Intra-arterial therapies for hepatocellular carcinoma: where do we stand? Ann Surg Oncol. 2010;17:1234-1246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Lu W, Li YH, He XF, Zhao JB, Chen Y, Mei QL. Necrosis and apoptosis in hepatocellular carcinoma following low-dose versus high-dose preoperative chemoembolization. Cardiovasc Intervent Radiol. 2008;31:1133-1140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Wu XZ, Xie GR, Chen D. Hypoxia and hepatocellular carcinoma: The therapeutic target for hepatocellular carcinoma. J Gastroenterol Hepatol. 2007;22:1178-1182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 152] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 16. | Wang W, Shi J, Xie WF. Transarterial chemoembolization in combination with percutaneous ablation therapy in unresectable hepatocellular carcinoma: a meta-analysis. Liver Int. 2010;30:741-749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Vogl TJ, Zangos S, Balzer JO, Nabil M, Rao P, Eichler K, Bechstein WO, Zeuzem S, Abdelkader A. [Transarterial chemoembolization (TACE) in hepatocellular carcinoma: technique, indication and results]. Rofo. 2007;179:1113-1126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Vogl TJ, Naguib NN, Nour-Eldin NE, Rao P, Emami AH, Zangos S, Nabil M, Abdelkader A. Review on transarterial chemoembolization in hepatocellular carcinoma: palliative, combined, neoadjuvant, bridging, and symptomatic indications. Eur J Radiol. 2009;72:505-516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 19. | Jin Z, El-Deiry WS. Overview of cell death signaling pathways. Cancer Biol Ther. 2005;4:139-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 730] [Cited by in F6Publishing: 848] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 20. | Marsden VS, O'Connor L, O'Reilly LA, Silke J, Metcalf D, Ekert PG, Huang DC, Cecconi F, Kuida K, Tomaselli KJ. Apoptosis initiated by Bcl-2-regulated caspase activation independently of the cytochrome c/Apaf-1/caspase-9 apoptosome. Nature. 2002;419:634-637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 429] [Cited by in F6Publishing: 433] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 21. | Ducasse E, Giannakakis K, Chevalier J, Dasnoy D, Puppinck P, Speziale F, Fiorani P, Faraggiana T. Dysregulated apoptosis in primary varicose veins. Eur J Vasc Endovasc Surg. 2005;29:316-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798-4811. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1537] [Cited by in F6Publishing: 1584] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 23. | Arnoult D, Parone P, Martinou JC, Antonsson B, Estaquier J, Ameisen JC. Mitochondrial release of apoptosis-inducing factor occurs downstream of cytochrome c release in response to several proapoptotic stimuli. J Cell Biol. 2002;159:923-929. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 249] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 24. | Chipuk JE, Green DR. Do inducers of apoptosis trigger caspase-independent cell death? Nat Rev Mol Cell Biol. 2005;6:268-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 248] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 25. | Fox R, Aubert M. Flow cytometric detection of activated caspase-3. Methods Mol Biol. 2008;414:47-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Brunelle JK, Chandel NS. Oxygen deprivation induced cell death: an update. Apoptosis. 2002;7:475-482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 72] [Reference Citation Analysis (1)] |
| 27. | Said TM, Paasch U, Glander HJ, Agarwal A. Role of caspases in male infertility. Hum Reprod Update. 2004;10:39-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 166] [Article Influence: 8.3] [Reference Citation Analysis (0)] |