Copyright
©2011 Baishideng Publishing Group Co.
World J Gastroenterol. Sep 14, 2011; 17(34): 3899-3911
Published online Sep 14, 2011. doi: 10.3748/wjg.v17.i34.3899
Published online Sep 14, 2011. doi: 10.3748/wjg.v17.i34.3899
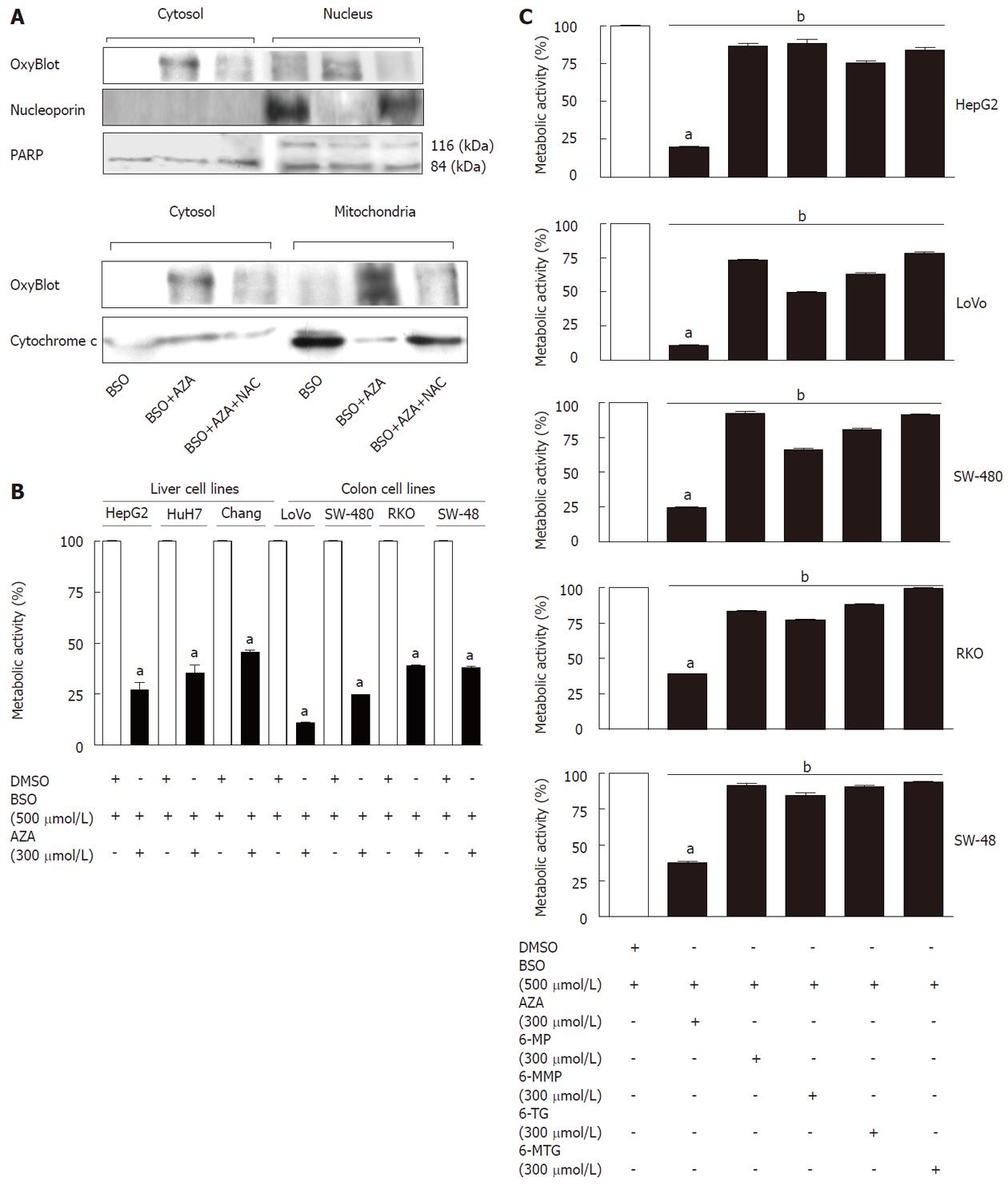
Figure 2 Effect of the treatment with thiopurines plus buthionine sulfoximine in cancerous cell lines.
A: Western blotting of several proteins from enriched fractions of cytosol, nucleus and mitochondria obtained from HepG2 cells pretreated with buthionine sulfoximine (BSO) (500 μmol/L, 24 h), or pretreated with BSO (500 μmol/L, 24 h) and then cotreated with azathioprine (AZA) (300 μmol/L) for 6 h with or without N-acetylcysteine (NAC) (1.5 mmol/L); B: Metabolic activity (by MTT assay) in different cancer cell lines from liver and colon pretreated with BSO (24 h) and then cotreated with dimethyl sulfoxide (DMSO) or with AZA for 12 h; C: Metabolic activity (by MTT assay) in different cell lines pretreated with BSO (24 h) and then cotreated with AZA or with different thiopurines for 12 h. Significant differences with respect to control (DMSO plus BSO) were statistically analyzed by analysis of variance with the Bonferroni post hoc test. aP < 0.001, BSO plus AZA vs control. bP < 0.001, BSO plus AZA vs BSO plus different thiopurines.
- Citation: Hernández-Breijo B, Monserrat J, Ramírez-Rubio S, Cuevas EP, Vara D, Díaz-Laviada I, Fernández-Moreno MD, Román ID, Gisbert JP, Guijarro LG. Preclinical evaluation of azathioprine plus buthionine sulfoximine in the treatment of human hepatocarcinoma and colon carcinoma. World J Gastroenterol 2011; 17(34): 3899-3911
- URL: https://www.wjgnet.com/1007-9327/full/v17/i34/3899.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i34.3899