Copyright
©2011 Baishideng Publishing Group Co.
World J Gastroenterol. Sep 14, 2011; 17(34): 3899-3911
Published online Sep 14, 2011. doi: 10.3748/wjg.v17.i34.3899
Published online Sep 14, 2011. doi: 10.3748/wjg.v17.i34.3899
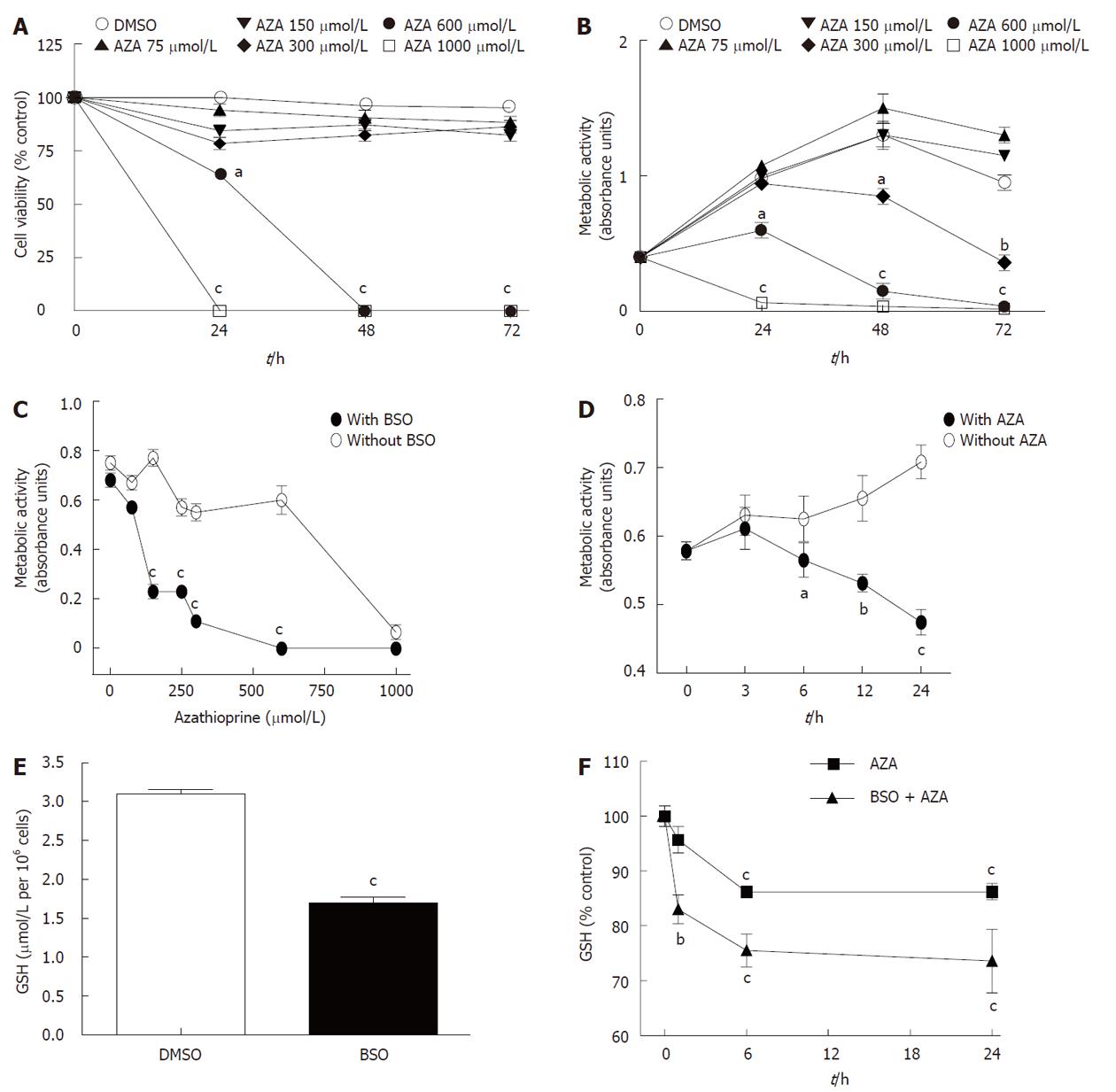
Figure 1 Effect of azathioprine treatment in HepG2 cells and sensitization of HepG2 cells by buthionine sulfoximine pretreatment.
A: Cell viability (by trypan blue exclusion assay); B: Metabolic activity (by MTT assay) of HepG2 cells treated at different times with dimethyl sulfoxide (DMSO) or azathioprine (AZA) at different concentrations; C: Metabolic activity of cultured HepG2 cells pretreated for 24 h without or with buthionine sulfoximine (BSO) (500 μmol/L) and then cotreated with AZA at different concentrations for 24 h, or D with AZA (100 μmol/L) at different times; E: Glutathione (GSH) content of HepG2 cells pretreated for 24 h with DMSO or BSO (500 μmol/L); F: GSH content of HepG2 cells pretreated for 24 h with DMSO or BSO (500 μmol/L) and then cotreated with AZA (300 μmol/L) for different times. The significant differences with respect to control were statistically analyzed by analysis of variance with the Bonferroni post hoc test. aP < 0.05; bP < 0.01; cP < 0.001.
- Citation: Hernández-Breijo B, Monserrat J, Ramírez-Rubio S, Cuevas EP, Vara D, Díaz-Laviada I, Fernández-Moreno MD, Román ID, Gisbert JP, Guijarro LG. Preclinical evaluation of azathioprine plus buthionine sulfoximine in the treatment of human hepatocarcinoma and colon carcinoma. World J Gastroenterol 2011; 17(34): 3899-3911
- URL: https://www.wjgnet.com/1007-9327/full/v17/i34/3899.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i34.3899