Published online Jul 21, 2011. doi: 10.3748/wjg.v17.i27.3267
Revised: February 15, 2011
Accepted: February 22, 2011
Published online: July 21, 2011
We report a rare case of duodenal pseudolymphoma without any symptoms. The lesion located in front of the head of the pancreas was found accidentally during a medical examination. The findings of computed tomography and positron emission tomography-computed tomography suggested a stromal tumor or malignant lymphoma. Surgical resection was performed. The lesions were pathologically diagnosed as duodenal pseudolymphoma.
- Citation: Huang YH, Long TZ, Xiao ZY, Ye H, Wan YL, Wang J. Duodenal pseudolymphoma: A case report and review of literature. World J Gastroenterol 2011; 17(27): 3267-3270
- URL: https://www.wjgnet.com/1007-9327/full/v17/i27/3267.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i27.3267
Pseudolymphoma is rare, and is thought to be a benign nodule lesion characterized by marked proliferation of polyclonal and non-neoplastic lymphoid cells forming follicles and active germinal centers. It is difficult to differentiate pseudolymphoma from a malignant tumor, and the accurate diagnosis of pseudolymphoma depends on histopathological examinations. Pseudolymphoma may be found in various organs, including the skin, orbit, thyroid,breast, lung, stomach, liver, small intestine, spleen, pancreas, kidney, uterus and testis[1,2]. However, to our knowledge, duodenal pseudolymphoma has never been reported before. We report a case of duodenal pseudolymphoma and review the literature below.
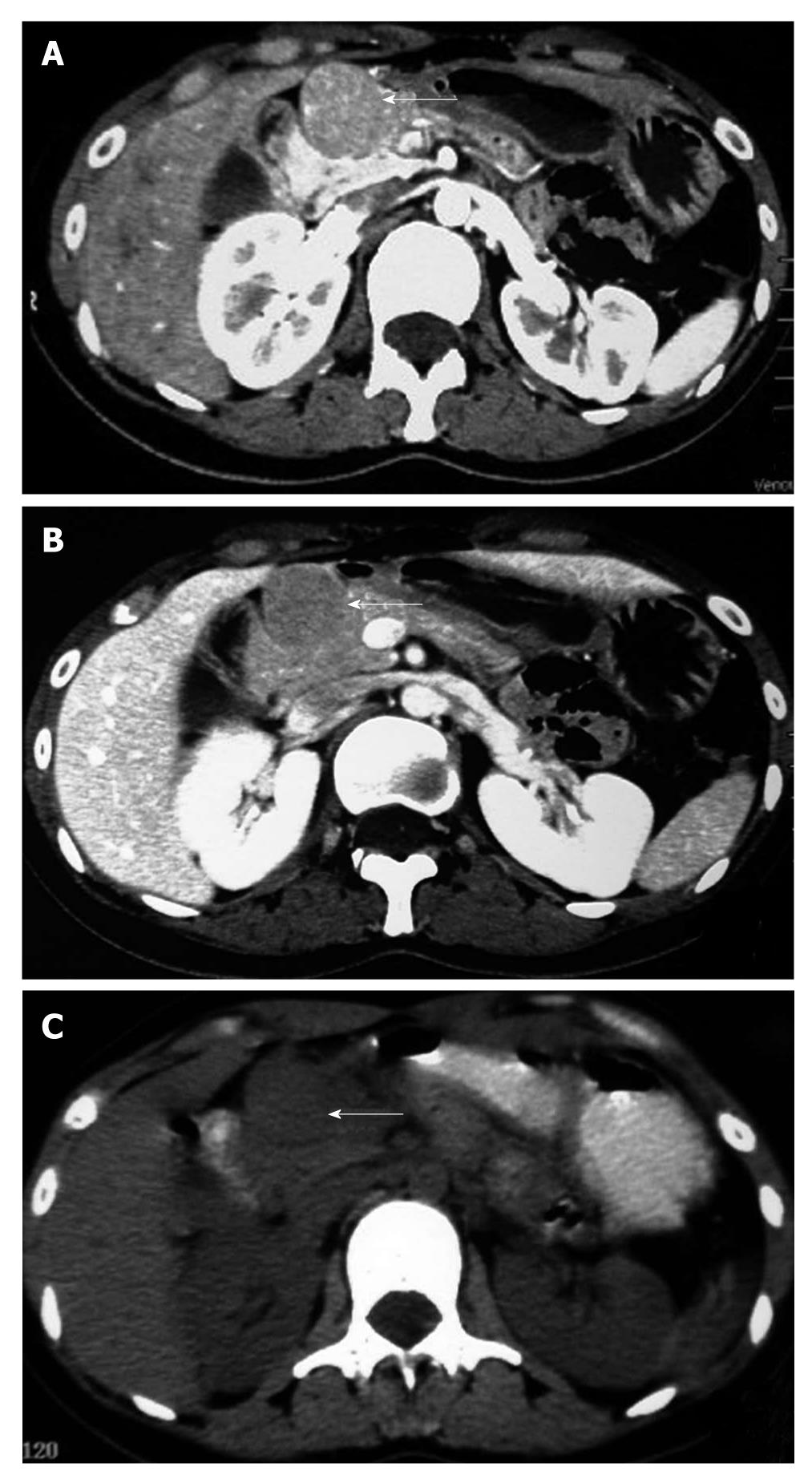
A 23-year-old woman underwent a routine medical examination, during which a space-occupying lesion was found in front of the head of the pancreas by ultrasonography (USG) on April 28, 2010. Computed tomography (CT) performed on May 4th revealed several isodense masses in front of the pancreatic head. The largest one was 30 mm in diameter and well-defined, and the surrounding pancreas, pylorus and duodenal bulb appeared compressed. There was no significant invasion, and it was strongly enhanced after injection of contrast medium. Pancreas was of uniform density without a significant space-occupying lesion. A para-aortic lymph node was seen, approximately 5 mm × 8 mm in size, well-defined, moderately enhanced in arterial phase. The CT scan diagnosis was metastatic stromal tumor (Figure 1A and B); white blood cell count 7.5 × 109/L and hemoglobin 92 g/L, and platelets 419 × 109/L; carcinoembryonic antigen 0.58 ng/mL (normal, 0.00-5.00) and carbohydrate antigen 8.64 U/mL (normal, 0.00-5.30). The patient was asymptomatic and physical examination revealed no abnormalities. She was admitted to our hospital for further treatment on May 17th, and laboratory data on admission including liver function, renal function and electrolyte tests were all unremarkable. PET-CT performed on May 25th demonstrated multiple low-density nodules behind the gastric antrum and in front of the pancreas head. PET regions showed a non-uniform abnormal focus of uptake. Standard uptake value (SUV) was about 2.9-5.8. The pancreas, skeleton, muscle and tissue showed no obvious abnormalities. There was no retroperitoneal and pelvic lymph node enlargement. PET-CT diagnosis was stromal tumor or malignant lymphoma (Figure 1C).
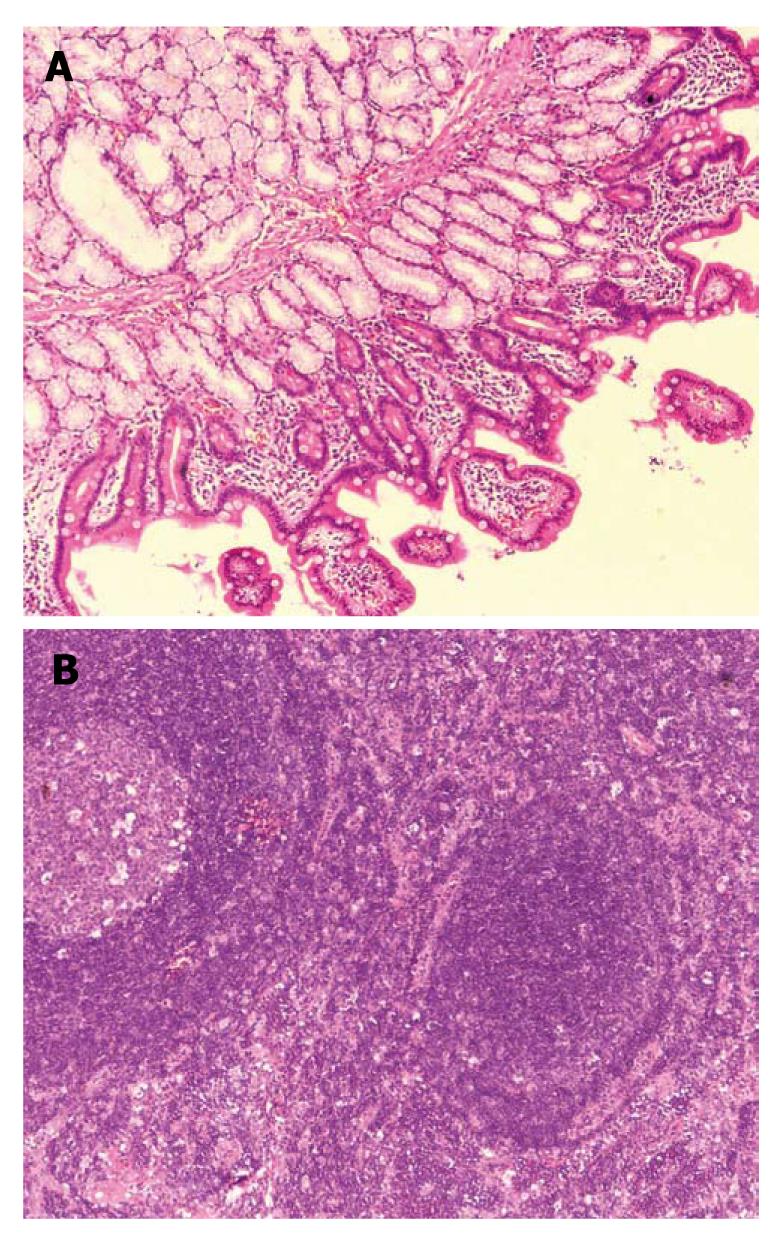
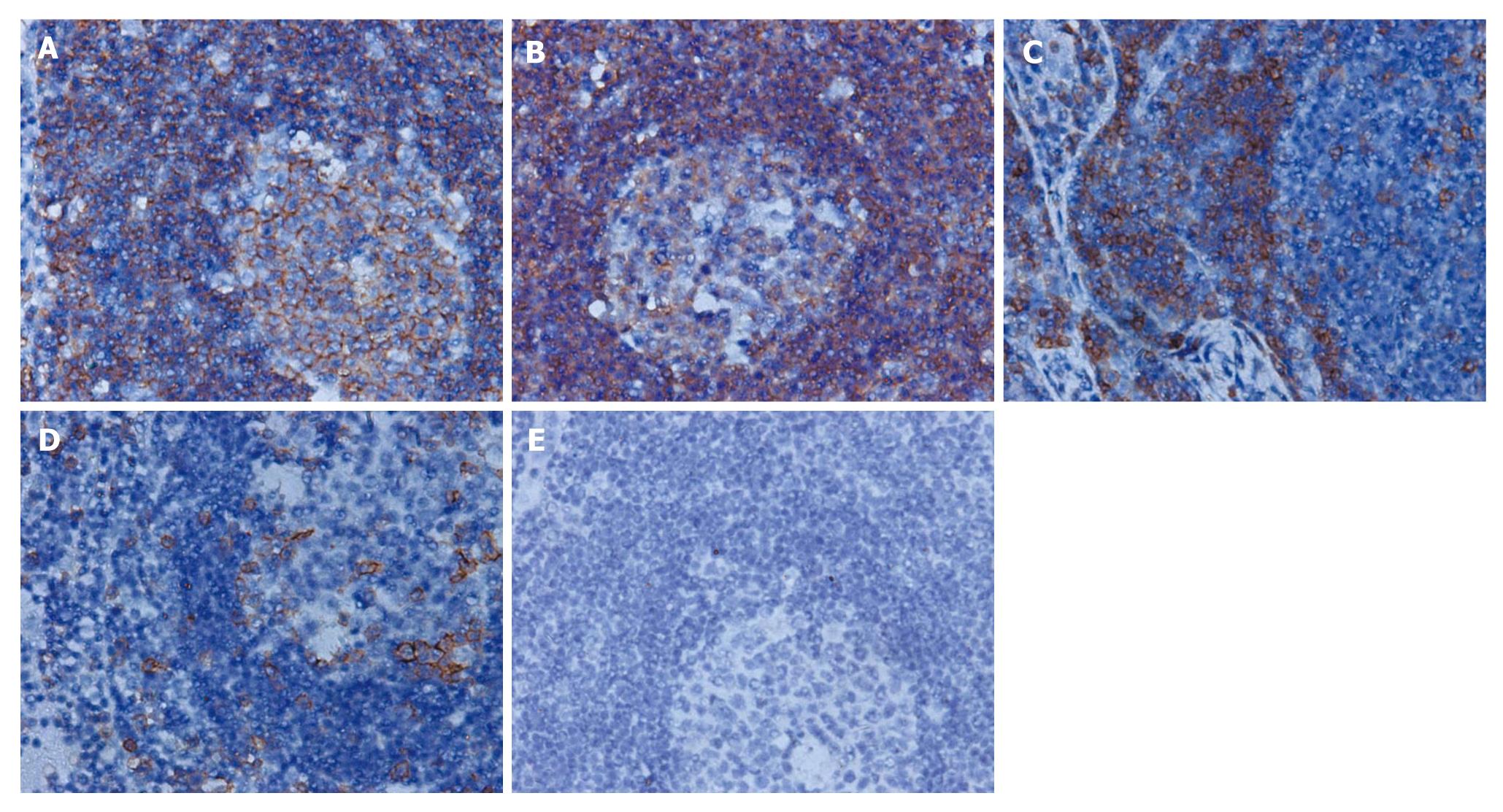
Laparotomy was performed on May 26th. The tumor was found located in the duodenal bulb with extended growth from the back to the bottom of the intestinal wall, and firmly attached to the head of the pancreas and the root of the mesocolon. There were several hard enlarged lymph nodes located near the root of middle colic vessel and behind the head of pancreas about 10-30 mm in diameter. Lymph nodes near the transverse colon were resected for frozen biopsy, which showed that lymph nodes had chronic inflammation and lymphoid hyperplasia. Due to the limitation of the frozen biopsy and the general form of the lymph nodes and the tumor growth pattern, we could not completely rule out the possibility of malignancy. Thus the complete resection of the tumor and enlarged lymph nodes was performed. The postoperative pathological examination report suggested lymph nodes with chronic inflammation and lymphoid hyperplasia. Duodenal pseudolymphoma was confirmed (Figure 2A and B). Immunohistochemical staining for CD79a, L-26, CD3 and UCHL-1 was positive, and for TDT it was negative (Figure 3A-E). The patient was discharged 10 d after surgery and showed no sign of recurrence in the follow-up of five months.
Pseudolymphoma, also named reactive lymphoid hyperplasia, was first described in 1963 by Saltzsein[3], and since then cases of pseudolymphoma of different organs have been successively reported although it is a rare disease.
At present, the exact pathogenesis of pseudolymphoma remains unknown. It has been reported that drugs (Phenytoin, Carbamazepine, Felodipine), foreign antigens (trauma, tattoo dyes and immunization), infection and sunlight allergy can cause cutaneous pseudolymphoma[4]. Autoimmune diseases (Sjogren’s syndrome and thyroiditis) are thought to be associated with pulmonary and hepatic pseudolymphoma[5]. Helicobacter pylori infection plays an important role in the etiology of gastric pseudolymphoma[6].Our patient had no obvious predisposing factors. She was asymptomatic and physical examination result was not remarkable. It was accidentally discovered during a routine USG. Therefore, more data are needed for further studies on the risk factors and onset mechanism of pseudolymphoma.
The preoperative diagnosis of pseudolymphoma is difficult. It should be differentiated from malignant lymphoma and other malignant tumors. Gastric pseudolymphoma is easily misdiagnosed as gastric cancer on barium meal and gastroscopy examinations. Radiologically, hepatic pseudolymphoma has been often misdiagnosed as hepatocellular carcinoma. The diagnosis of our case on imaging studies could not be discriminated from stromal tumor and malignant lymphoma and a final diagnosis relied on the pathological examinations. Microscopically, pseudolymphoma is characterized by the presence of hyperplastic lymphoid follicles with germinal centers, consisting of polymorphic and polyclonal cell populations and aggregations of mature lymphocytes, mature plasma cells, macrophages and other types of inflammatory cells. Immunohistochemical tests showed negative results for bcl-2. Immunoglobulin heavy chains (IgH) and T cell receptor (TCR) gene rearrangement analysis revealed polyclonal proliferation or no gene rearrangement[7]. In malignant lymphoma, lymphoid follicles and germinal centers were not found, cellular atypia was significant, caryocinesia was easily observed and the infiltrating lymphocytes were monoclonal. Muria et al[8] considered that it is not possible to rule out the diagnosis of pseudolymphoma with the absence of germinal centers and that the pseudolymphoma has mainly mature lymphocytes while lymphoma has non-mature lymphocytes, so they were able to be distinguished according to the differentiation degree of the infiltrating lymphocytes. Takeshita et al[9]reported that the Southern blot analysis showed immunoglobulin heavy chain JH and light chain J-κ clone rearrangement, which helped distinguish pseudolymphoma from malignant lymphoma. Therefore, in addition to pathological examination, immune phenotype and gene rearrangements can also be used to further confirm the diagnosis of pseudolymphoma. On the histopathological examination, various sized and shaped lymphoid follicles with reactive germinal center formation were seen in our patient. Infiltrating polymorphic lymphocytes and polyclonal cell populations were composed of lymphoblastoid cells, plasma cells and macrophages without atypical and obvious caryocinesia.
Immunohistochemical studies revealed polyclonality, lymphoid cells positive for L-26 and CD 79a (B cell marker), mainly aggregated in the germinal centers, while those positive for CD3 and UCHL-1 (T cell marker) were present around the germinal centers. These findings were compatible with reactive lymphoid hyperplasia. Positive TDT has been often seen in lymphoblastic lymphoma. In our case, immunohistochemical staining for TDT was negative, which further confirmed the diagnosis of pseudolymphoma.
Although pseudolymphoma is generally considered to be a benign tumor, there is a risk of malignant transformation into lymphoma. It has been reported that some cases such as cutaneous, pulmonary, gastric and hepatic pseudo lymphomas initially diagnosed as pseudolymphoma had transformed into lymphoma several years later[10,11]. So surgical resection remains the best option for treatment of pseudolymphoma at present. Intraoperative frozen biopsy can be used to diagnose pseudolymphoma and if cell variation within frozen section makes the feature of the lesion unclear, it can be treated according to the therapeutic principle of malignant tumors. Whether it is necessary to perform postoperative adjuvant chemotherapy remains inconclusive, as pseudolymphoma is mainly composed of mature lymphocytes, and less sensitive to chemotherapy. The lesions are not easily dissipated, and it has been reported that pseudolymphoma after chemotherapy still transformed into malignant lymphoma. Therefore, more cases should be accumulated to determine whether chemotherapy is necessary. Duodenal pseudolymphoma has never been reported before and its prognosis is not so clear. Although our patient had an early diagnosis, complete tumor resection, enlarged lymph nodes dissection and uneventful postoperative course, she must be followed up carefully because of the possible later development of malignant lymphoma.
Peer reviewer: Jon C Gould, MD, FACS, Associate Professor of Surgery, University of Wisconsin School of Medicine and Public Health, 600 Highland Avenue, H4/726, Madison, WI 53792, United States
S- Editor Tian L L- Editor Ma JY E- Editor Ma WH
| 1. | Ganzer R, Burger M, Woenckhaus M, Wieland WF, Blana A. A patient with testicular pseudolymphoma - a rare condition mimicking malignancy: a case report. J Med Case Reports. 2007;1:71. [Cited in This Article: ] |
| 2. | Okada T, Mibayashi H, Hasatani K, Hayashi Y, Tsuji S, Kaneko Y, Yoshimitsu M, Tani T, Zen Y, Yamagishi M. Pseudolymphoma of the liver associated with primary biliary cirrhosis: a case report and review of literature. World J Gastroenterol. 2009;15:4587-4592. [Cited in This Article: ] |
| 3. | Saltzstein SL. Pulmonary malignant lymphomas and pseudolymphomas: classification, therapy, and prognosis. Cancer. 1963;16:928-955. [Cited in This Article: ] |
| 4. | Kabashima R, Orimo H, Hino R, Nakashima D, Kabashima K, Tokura Y. CD30-positive T-cell pseudolymphoma induced by amlodipine. J Eur Acad Dermatol Venereol. 2008;22:1522-1524. [Cited in This Article: ] |
| 5. | Song MK, Seol YM, Park YE, Kim YS, Lee MK, Lee CH, Jeong YJ. Pulmonary nodular lymphoid hyperplasia associated with Sjögren’s syndrome. Korean J Intern Med. 2007;22:192-196. [Cited in This Article: ] |
| 6. | Chen XY, Liu WZ, Shi Y, Zhang DZ, Xiao SD, Tytgat GN. Helicobacter pylori associated gastric diseases and lymphoid tissue hyperplasia in gastric antral mucosa. J Clin Pathol. 2002;55:133-137. [Cited in This Article: ] |
| 7. | Machida T, Takahashi T, Itoh T, Hirayama M, Morita T, Horita S. Reactive lymphoid hyperplasia of the liver: a case report and review of literature. World J Gastroenterol. 2007;13:5403-5407. [Cited in This Article: ] |
| 8. | Miura H, Taira O, Uchida O, Kajiwara N, Kato H. Primary pulmonary lymphoma diagnosed by gene rearrangement: report of a case. Surg Today. 1996;26:457-460. [Cited in This Article: ] |
| 9. | Takeshita T, Miyaji N, Churei H, Moriyama T, Ogita M, Nakajo M, Oyama T, Shimokawahara H, Nakamura T. A case of pulmonary pseudolymphoma: five years’ roentgenographic observation. Radiat Med. 1995;13:243-246. [Cited in This Article: ] |
| 10. | Okubo H, Maekawa H, Ogawa K, Wada R, Sekigawa I, Iida N, Maekawa T, Hashimoto H, Sato N. Pseudolymphoma of the liver associated with Sjögren’s syndrome. Scand J Rheumatol. 2001;30:117-119. [Cited in This Article: ] |
| 11. | Sangueza OP, Yadav S, White CR Jr, Braziel RM. Evolution of B-cell lymphoma from pseudolymphoma. A multidisciplinary approach using histology, immunohistochemistry, and Southern blot analysis. Am J Dermatopathol. 1992;14:408-413. [Cited in This Article: ] |