Published online Jul 21, 2011. doi: 10.3748/wjg.v17.i27.3192
Revised: June 24, 2011
Accepted: July 1, 2011
Published online: July 21, 2011
Diagnosis of chronic inflammatory bowel diseases (IBD) is based on a combination of clinical symptoms, laboratory tests and imaging data. Imaging of the morphological characteristics of IBD includes the assessment of mucosal alterations, transmural involvement and extraintestinal manifestations. No single imaging technique serves as a diagnostic gold standard to encompass all disease manifestations. Ultrasound, computed tomography (CT) or magnetic resonance imaging (MRI) allow cross-sectional imaging of the transmural alterations and extraintestinal manifestations. While in the USA the technique of choice is CT, in Europe the focus is more on MRI and ultrasound (US). Most patients with chronic IBD are diagnosed at a young age. After baseline diagnosis many of these young patients have to undergo repetitive imaging procedures during the variable clinical course of the disease, characterized by alternate periods of remission and active disease, and in monitoring the response to treatment. US has the advantage of being noninvasive, less costly, and easily repeatable, and thus can be very useful in following up patients with IBD. In addition, rising concern about radiation exposure in young adults indicates the demand for radiation-sparing techniques like US and MRI. This article focuses on the current clinical practice of US in IBD, describing the current technologies used in transabdominal intestinal US and the characteristic sonographic findings in Crohn´s disease and ulcerative colitis.
- Citation: Strobel D, Goertz RS, Bernatik T. Diagnostics in inflammatory bowel disease: Ultrasound. World J Gastroenterol 2011; 17(27): 3192-3197
- URL: https://www.wjgnet.com/1007-9327/full/v17/i27/3192.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i27.3192
Ultrasound (US) for inflammatory bowel diseases (IBD) requires high-frequency (5-17 MHz) linear array probes. High-frequency linear-array probes provide increased spatial resolution of the intestinal wall, which is essential for the assessment of wall diameter and wall layer discrimination. In modern high-frequency probes the creation of special modulated US pulses in transmission results in greater penetration of high frequency US. Compounding technology allows image reconstruction using signal responses from different frequencies or from viewing indifferent directions that results in an increase in contrast resolution and border definition of bowel wall architecture. Color or power Doppler imaging and contrast-enhanced US (CEUS) provide detailed information on mural and extraintestinal vascularity, which reflect inflammatory disease activity.
Conventional transabdominal US with a conventional 3.5-5 MHz convex probe is recommended prior to high-frequency US of the intestinal tract so as not to overlook underlying extraintestinal causes of abdominal discomfort. Special attention should be paid to the lower abdomen (urogenital tract) and the individual patient’s pain location. High-frequency US of the intestinal tract requires extra time and patience of the examiner. With the exception of emergency situations the standard US examination should be performed preprandial in the morning or at least after 4 h fasting to diminish peristaltic movements and the amount of intraluminal air. Gradual compression of the bowel with the US probe helps to reduce intraluminal air. The application of intraluminal fluid as used in bowel preparation for colonoscopy or the use of enteral contrast medium[1-4] has been shown to improve the delineation of the wall architecture and the detection of jejunal and colonic lesions in patients with IBD, but these more sophisticated techniques have not been transferred into routine clinical practice. For US diagnosis of IBD, an understanding of the anatomical location of Crohn’s disease (CD) and ulcerative colitis (UC), and of the more difficult or non accessible parts of the small and large bowel is essential for a systemic US approach to the patient. Transabdominal high-frequency US does not provide a continuous and complete examination of the small and large bowel. The ileocecal region and the sigmoid colon can be identified in all patients. The left and right colon can be adequately evaluated in most of patients. The colonic flexures (especially the left flexure) are more difficult to visualize due to their cranial position and ligamentous fixation to the diaphragm. The colon transversum can be identified in most patients, but complete examination is not easy to achieve because of its variable anatomy. The rectum and anal region cannot be visualized accurately by the transabdominal route due to their pelvic location. Transperineal US is useful in the evaluation of the perianal region and the distal rectum[5]. A proposal for a systematic approach in IBD patients could be to start in the left lower abdomen with transverse scans using the left iliac artery and vein as landmarks to visualize the sigmoid colon. The sigmoid colon can be easily identified by its prominent hypoechoic muscle layer (muscularis propria). Examination of the left colon can then be adequately performed by continuous scanning from the rectosigmoid transition along the colon descendens upwards to the left costal arch. Gradual compression is recommended to follow the left-sided colon along. The next step of a systematic examination could be visualization of the ileocecal region with transverse scans in the right lower abdomen using the right iliac artery and vein as landmarks and gradual compression. A variable location of ileocecal region in the right middle abdomen (also a frequent location of the neoterminal ileum after surgical resection) can be identified after manual palpation of the right spina iliaca anterior superior as a landmark. Moving the US probe in transverse sections from the right spina iliaca anterior superior upwards to the right costal arch with graded compression helps to find the lumen of the colon ascendens with its characteristic broad lumen (hyperechoic air filled lumen or hypoechoic fluid filled lumen). The cecum can easily be found by turning the probe towards a longitudinal position to follow the colon ascendens downwards until the broad luminal echo disappears. The distal part of the ileum can also be identified by transverse scanning from the cecum towards the middle and lower parts of the abdomen, again using the right iliac artery and vein and the urinary bladder as landmarks. Whereas the distal part of the small intestine (terminal ileum) can be evaluated in all patients, a complete and continuous evaluation of the proximal parts of the ileum and jejunum is not possible due to multiple overlying bowel loops. However, for a systematic approach to the proximal small intestine, four scanning positions in the upper and lower, right and left abdominal quadrants are recommended as final steps of a systematic approach to search for thickened intestinal wall segments.
With the use of high US frequencies in the range from 7.5 MHz to 17 MHz, the wall of the intestine usually exhibits five different layers (Table 1). The small and large bowel can usually be distinguished in various stages of filling during movement by scanning the haustra of the colon and/or the circular folds of Kerckring in the small intestine. Measurement of the wall thickness is crucial for the diagnosis of IBD. Discrepancies in the measurements are mainly due to the presence or absence of graded compression during the examination by the operator in addition to various technical causes (US frequency, equipment). With modern high-frequency linear array probes the normal intestinal wall thickness is generally ≤ 3 mm (using mild compression) ranging from small diameters in the jejunum, ileum and proximal colon to larger diameters in the sigmoid colon (due to the hypertensive function of the sigmoid zone). Physiological contraction of the intestine leading to a thickened wall segment may cause misinterpretation, therefore bowel motions have to be taken into account before measurements are performed. In addition to wall thickening, echomorphology (integrity of wall architecture) and surrounding structures have also to be considered in the interpretation of the intestinal wall diameter.
| Layer echogenicity | Anatomic structure |
| Hypoechoic (fluid) or hyperechoic (air) lumen | |
| Hyperechoic entrance | Transition lumen/mucosa |
| Hypoechoic | Mucosa |
| Hyperechoic | Submucosa |
| Hypoechoic | Muscularis propria |
| Hyperechoic | Transition muscularis propria/serosa, surrounding structures (fat, peritoneal wall) |
CD can be localized in any part of the gastrointestinal tract, although the main location is the terminal ileum. Small intestinal localization of the disease is found in 30%-40% of patients with CD (with involvement of the terminal ileum in 90%), and 40%-55% of the patients show an ileum and colonic localization. Only in a minority of patients (15%-25%) is colonic localization only observed. A systematic examination in patients with IBD should include complete scanning of the ileocecal region and sigmoid colon as well as the remaining parts of the colon, and evaluation of the small intestine (in sections) and surrounding mesenteric structures.
The most widely used diagnostic criterion for the diagnosis of IBD is bowel wall thickening with increased vascularization (with maintenance or loss of wall stratification).
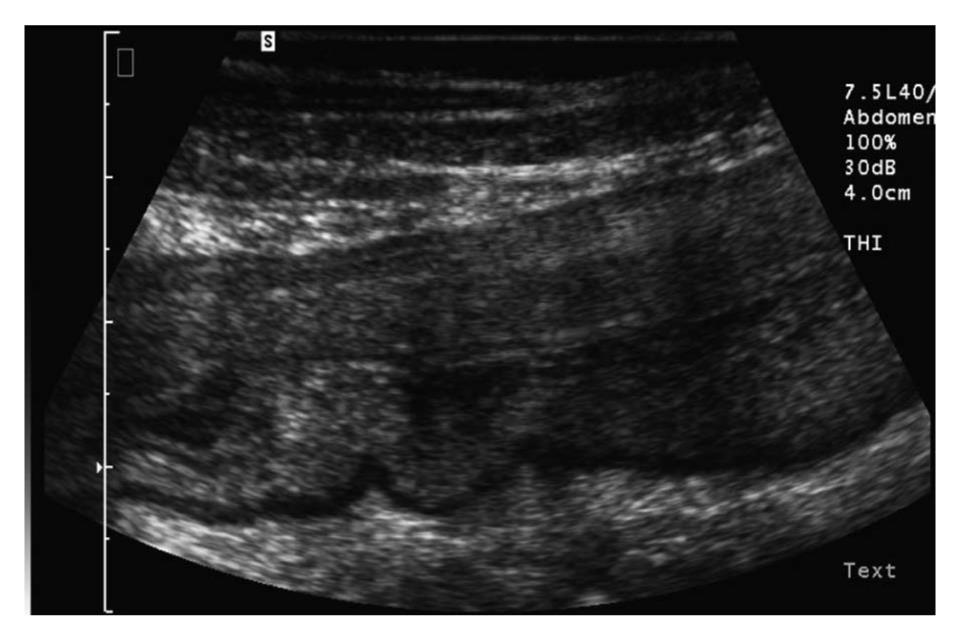
In most studies, the bowel is considered to be thickened when the wall diameter exceeds 3 mm (Figure 1). The diagnostic accuracy of different cut-off values in CD were compared in a metaanalysis by Fraquelli et al[6]. Sensitivity and specificity of 88% and 93%, respectively, were achieved when a bowel wall thickness threshold greater than 3 mm was used, and sensitivity and specificity of 75% and 97%, respectively, were achieved when a threshold greater than 4 mm was used. Several studies report a relation between bowel wall thickness and clinical disease activity using the Crohn´s Disease Activity Index (CDAI) or Harvey Bradshaw Index (HBI) at initial diagnosis and during the clinical course of CD and in relation to endoscopic findings[7-9].
Bowel ultrasonographic signs used in CD can be standardized as most show a fair to good reproducibility. In particular, bowel wall thickness, the most relevant parameter for CD detection, showed an excellent reproducibility[10]. Color or power Doppler imaging of the vascularity of thickened wall segments using semiquantitative scores has proved useful in the distinction between remission or the active phase in CD and correlated with the clinical and endoscopic activity scores in adults[9,11] and children[12]. A reduction in wall thickness and mural vascularity (color Doppler imaging) could be shown in patients with a positive clinical-biological response to anti-tumor necrosis factor-α induction therapy in a small study in 24 patients. Maintenance of wall thickness and increased mural vascularity were seen in all non-responders (n = 7). Sonographic normality (defined as wall thickness ≤ 3 mm and Doppler flow grade = 0) was only seen in 5 of 17 patients with a positive clinical-biological therapeutic response (29%)[13]. Most studies on Doppler US include a subjective semiquantitative assessment of mural vascularity, e.g. grade 0 = no vascular signal, grade 1 = barely visibly signals, grade 2 = moderate vascularity, grade 3 = marked vascularity. Currently there is no objective scale to determine the degree of disease activity on Doppler US.
CEUS is the most sensitive technique to visualize microperfusion and has been shown to be superior to conventional color or power Doppler imaging in determining tumor vascularity[14]. Currently, the clinical value of CEUS in IBD is not well defined. Most studies on CEUS have been feasibility or pilot studies.
In a small study (21 patients) with histologically confirmed CD and bowel wall diameters > 5 mm, contrast enhancement was observed after a mean of 13.4 s (± 4.2 s; range, 7-19 s) with a maximum vascularity after 30 s[15].. In addition, the length of contrast-enhanced bowel segments in US correlated significantly with the length of thickened bowel segments in magnetic resonance imaging (MRI)[16]. In a retrospective analysis, the assessment of the bowel wall vascularization in CD was performed using quantification software, indicating that CEUS data can not only be analyzed in a semi-quantitative way, but also in a reproducible, quantitative manner[17]. Using time-intensity curves, patients with CD showed a maximum enhancement 36 s after injection with 9 dB (range, 5.9-13.2 dB), while healthy volunteers reached the maximum level of 2.8 dB (range, 2-3.8 dB) after 23 s (P < 0.05)[18].
Correlation of CEUS with disease activity (endoscopy, histology, CDAI) indicate that active disease can be identified by CEUS)[19,20]. Characterization of the enhancement pattern in relation to wall thickness was shown to distinguish CD patients with active and inactive disease[21]. Lower levels of bowel contrast enhancement were observed in some patients who responded to anti-inflammatory treatment[22,23], as was shown in power Doppler imaging a few years ago. CEUS may be a useful method to assess the therapeutic effectiveness of specific medical anti-inflammatory treatment in patients with CD, or to differentiate inflammatory from fibrotic bowel wall changes. In addition, CEUS was suggested to be helpful in surgical management, in deciding upon conservative vs surgical treatment[24,25]. However, the role of CEUS in addition to conventional Doppler imaging is not yet well defined in clinical studies. Pilot studies on CEUS are promising, but studies on larger patient numbers, including the objective contrast enhancement score are needed before CEUS can be considered a clinical useful tool.
Patients with CD had significantly higher portal vein and mesenteric flow and a lower resistance index than controls[26]. Blood flow in the superior mesenteric artery (SMA) has shown an increase in the postprandial pulsatility index (PI) in remission[27] in Doppler US. A decrease in PI predicted a non responder to azathioprine therapy and clinical relapse in a 12-mo follow-up[28]. Contrast enhancement in the SMA and vein, and calculation of splanchnic transit time showed a reduction in transit time (4.0 s vs 6.9 s) in patients with active CD[29]. However, in our own experience splanchnic transit time measurement in CEUS was not correlated with clinical disease activity (HBI) in mild stages of CD.
In addition to the assessment of bowel thickness and increased mural vascularity, the surrounding structures (fat, lymph nodes, free fluid accumulation) may indicate a peri-testinal inflammatory reaction. Mesenteric fat hypertrophy correlated with biochemical and clinical activity of CD and with internal fistulas and increased bowel wall thickness. In quiescent CD, mesenteric hypertrophy does not appear to be a risk factor for relapse[30]. US is very sensitive for the detection of free fluid in CD[31]. The presence of regional lymph nodes shows only a weak correlation with clinical and biochemical CD activity[32]. Furthermore, the finding of mesenteric lymph nodes is non-specific and may reflect disease activity, but infectious intestinal diseases have to be excluded by stool and serologic tests.
Since US can find both intraluminal and peri-intestinal pathological features, it is a particularly valuable tool for the detection of complications of CD, such as stenosis, fistulas, and abscesses. Sensitivity and specificity for detecting fistulae in transabdominal US have been reported as 50%-89% and 90%-95%, respectively[33]. Sensitivity and specificity for detecting abscesses in transabdominal US is even higher with sensitivities of 71%-100% and specificities of 77%-94%[33-36]. In a series of 58 patients with CD, including 28 patients with bowel stenosis, 23 patients with fistulas and 10 patients with abscesses, high-resolution US showed a high diagnostic accuracy in comparison to clinical, endoscopic, radiological and operative findings[37]. The sensitivity, specificity, positive predictive and negative predictive values for US were 86%, 90%, 83% and 92%, respectively, for stenosis and 78%, 95%, 86% and 91%, respectively, for fistulas. The highest diagnostic accuracy was found for abscesses with sensitivity, specificity, positive predictive and negative predictive values 90%, 99%, 90% and 99%, respectively.
UC exclusively affects the colon with a predictable way of spreading from distal to proximal colon in a continuous manner. UC is classified by disease extent into proctitis, left-sided colitis and extensive colitis beyond the splenic flexure. A solitary rectal location of UC cannot be visualized accurately due to the pelvic location of the rectum. Mural stratification is preserved in most UC patients due to the superficial pattern of inflammation. The spatial resolution of US is not high enough to allow detection of mucosal pathology but bowel wall thickening is also a characteristic feature of UC[38].
The clinical role of US in UC is less well established as compared with CD. In contrast to CD, bowel thickening in UC could not be correlated with clinical disease activity in some studies[39,40]. However, compared with endoscopic findings, with an overall accuracy of 89% for US (bowel wall thickness > 3 mm and increased Doppler signal) and 73% for MRI (contrast enhancement in bowel wall) in identifying active IBD, the diagnostic accuracy was better in patients with UC than in patients with CD for both US and MRI[41].
Mucosal healing (MH) after short-term medical treatment is being considered as an important step in the therapeutic work-up of IBD patients due to the potential prognostic role of MH in predicting disease outcome. However, IBD patients are reluctant to be re-endoscopes during follow-up; therefore, there is a need for a non-invasive alternative index of MH which can replace endoscopy in clinical practice. In a prospective trial in 83 patients with UC with a follow-up of 15 mo, a high and consistent concordance between endoscopic and US scores was shown. In patients with UC, moderate-to-severe endoscopic and US scores at 3 mo were associated with a high risk of endoscopic activity at 15 mo, indicating that bowel US may be used as a surrogate of colonoscopy in assessing the short-term response of severe forms of UC to therapy. In addition, the US score and endoscopic score after 3 mo of steroid therapy predicted the outcome of disease at 15 mo[42,43].
Splanchnic flow measurements in the inferior mesenteric artery have been shown to be closely related to clinical and endoscopic disease activity in patients with UC[44,45]. In a small trial, CEUS showed entire bowel wall vascularity in correlation to clinical nonresponders to cytapheresis for steroid-refractory or -dependent UC[46]. So far CEUS is not routinely used in UC.
Transabdominal US is currently accepted as a clinically important first-line imaging technique in IBD in initial diagnosis and during the clinical course of the disease.
Peer reviewer: Luis Bujanda, PhD, Professor, Department of Gastroenterology, CIBEREHD, University of Country Basque, Donostia Hospital, Paseo Dr. Beguiristain s/n, 20014 San Sebastián, Spain
S- Editor Tian L L- Editor Cant MR E- Editor Ma WH
| 1. | Pallotta N, Tomei E, Viscido A, Calabrese E, Marcheggiano A, Caprilli R, Corazziari E. Small intestine contrast ultrasonography: an alternative to radiology in the assessment of small bowel disease. Inflamm Bowel Dis. 2005;11:146-153. [Cited in This Article: ] |
| 2. | Parente F, Greco S, Molteni M, Anderloni A, Sampietro GM, Danelli PG, Bianco R, Gallus S, Bianchi Porro G. Oral contrast enhanced bowel ultrasonography in the assessment of small intestine Crohn‘s disease. A prospective comparison with conventional ultrasound, x ray studies, and ileocolonoscopy. Gut. 2004;53:1652-1657. [Cited in This Article: ] |
| 3. | Bru C, Sans M, Defelitto MM, Gilabert R, Fuster D, Llach J, Lomeña F, Bordas JM, Piqué JM, Panés J. Hydrocolonic sonography for evaluating inflammatory bowel disease. AJR Am J Roentgenol. 2001;177:99-105. [Cited in This Article: ] |
| 4. | Limberg B, Osswald B. Diagnosis and differential diagnosis of ulcerative colitis and Crohn‘s disease by hydrocolonic sonography. Am J Gastroenterol. 1994;89:1051-1057. [Cited in This Article: ] |
| 5. | Maconi G, Ardizzone S, Greco S, Radice E, Bezzio C, Bianchi Porro G. Transperineal ultrasound in the detection of perianal and rectovaginal fistulae in Crohn‘s disease. Am J Gastroenterol. 2007;102:2214-2219. [Cited in This Article: ] |
| 6. | Fraquelli M, Colli A, Casazza G, Paggi S, Colucci A, Massironi S, Duca P, Conte D. Role of US in detection of Crohn disease: meta-analysis. Radiology. 2005;236:95-101. [Cited in This Article: ] |
| 7. | Calabrese E, Petruzziello C, Onali S, Condino G, Zorzi F, Pallone F, Biancone L. Severity of postoperative recurrence in Crohn‘s disease: correlation between endoscopic and sonographic findings. Inflamm Bowel Dis. 2009;15:1635-1642. [Cited in This Article: ] |
| 8. | Rigazio C, Ercole E, Laudi C, Daperno M, Lavagna A, Crocella L, Bertolino F, Viganò L, Sostegni R, Pera A. Abdominal bowel ultrasound can predict the risk of surgery in Crohn‘s disease: proposal of an ultrasonographic score. Scand J Gastroenterol. 2009;44:585-593. [Cited in This Article: ] |
| 9. | Drews BH, Barth TF, Hänle MM, Akinli AS, Mason RA, Muche R, Thiel R, Pauls S, Klaus J, von Boyen G. Comparison of sonographically measured bowel wall vascularity, histology, and disease activity in Crohn‘s disease. Eur Radiol. 2009;19:1379-1386. [Cited in This Article: ] |
| 10. | Fraquelli M, Sarno A, Girelli C, Laudi C, Buscarini E, Villa C, Robotti D, Porta P, Cammarota T, Ercole E. Reproducibility of bowel ultrasonography in the evaluation of Crohn‘s disease. Dig Liver Dis. 2008;40:860-866. [Cited in This Article: ] |
| 11. | Neye H, Voderholzer W, Rickes S, Weber J, Wermke W, Lochs H. Evaluation of criteria for the activity of Crohn‘s disease by power Doppler sonography. Dig Dis. 2004;22:67-72. [Cited in This Article: ] |
| 12. | Spalinger J, Patriquin H, Miron MC, Marx G, Herzog D, Dubois J, Dubinsky M, Seidman EG. Doppler US in patients with crohn disease: vessel density in the diseased bowel reflects disease activity. Radiology. 2000;217:787-791. [Cited in This Article: ] |
| 13. | Paredes JM, Ripollés T, Cortés X, Martínez MJ, Barrachina M, Gómez F, Moreno-Osset E. Abdominal sonographic changes after antibody to tumor necrosis factor (anti-TNF) alpha therapy in Crohn‘s Disease. Dig Dis Sci. 2010;55:404-410. [Cited in This Article: ] |
| 14. | Strobel D, Raeker S, Martus P, Hahn EG, Becker D. Phase inversion harmonic imaging versus contrast-enhanced power Doppler sonography for the characterization of focal liver lesions. Int J Colorectal Dis. 2003;18:63-72. [Cited in This Article: ] |
| 15. | Kratzer W, Schmidt SA, Mittrach C, Haenle MM, Mason RA, Von Tirpitz C, Pauls S. Contrast-enhanced wideband harmonic imaging ultrasound (SonoVue): a new technique for quantifying bowel wall vascularity in Crohn‘s disease. Scand J Gastroenterol. 2005;40:985-991. [Cited in This Article: ] |
| 16. | Pauls S, Gabelmann A, Schmidt SA, Rieber A, Mittrach C, Haenle MM, Brambs HJ, Kratzer W. Evaluating bowel wall vascularity in Crohn‘s disease: a comparison of dynamic MRI and wideband harmonic imaging contrast-enhanced low MI ultrasound. Eur Radiol. 2006;16:2410-2417. [Cited in This Article: ] |
| 17. | Girlich C, Jung EM, Iesalnieks I, Schreyer AG, Zorger N, Strauch U, Schacherer D. Quantitative assessment of bowel wall vascularisation in Crohn‘s disease with contrast-enhanced ultrasound and perfusion analysis. Clin Hemorheol Microcirc. 2009;43:141-148. [Cited in This Article: ] |
| 18. | Schreyer AG, Finkenzeller T, Gössmann H, Daneschnejad M, Müller-Wille R, Schacherer D, Zuber-Jerger I, Strauch U, Feuerbach S, Jung EM. Microcirculation and perfusion with contrast enhanced ultrasound (CEUS) in Crohn‘s disease: first results with linear contrast harmonic imaging (CHI). Clin Hemorheol Microcirc. 2008;40:143-155. [Cited in This Article: ] |
| 19. | Ripollés T, Martínez MJ, Paredes JM, Blanc E, Flors L, Delgado F. Crohn disease: correlation of findings at contrast-enhanced US with severity at endoscopy. Radiology. 2009;253:241-248. [Cited in This Article: ] |
| 20. | Migaleddu V, Scanu AM, Quaia E, Rocca PC, Dore MP, Scanu D, Azzali L, Virgilio G. Contrast-enhanced ultrasonographic evaluation of inflammatory activity in Crohn‘s disease. Gastroenterology. 2009;137:43-52. [Cited in This Article: ] |
| 21. | Serra C, Menozzi G, Labate AM, Giangregorio F, Gionchetti P, Beltrami M, Robotti D, Fornari F, Cammarota T. Ultrasound assessment of vascularization of the thickened terminal ileum wall in Crohn‘s disease patients using a low-mechanical index real-time scanning technique with a second generation ultrasound contrast agent. Eur J Radiol. 2007;62:114-121. [Cited in This Article: ] |
| 22. | Guidi L, De Franco A, De Vitis I, Armuzzi A, Semeraro S, Roberto I, Papa A, Bock E, Gasbarrini G, Fedeli G. Contrast-enhanced ultrasonography with SonoVue after infliximab therapy in Crohn‘s disease. Eur Rev Med Pharmacol Sci. 2006;10:23-26. [Cited in This Article: ] |
| 23. | Quaia E, Migaleddu V, Baratella E, Pizzolato R, Rossi A, Grotto M, Cova MA. The diagnostic value of small bowel wall vascularity after sulfur hexafluoride-filled microbubble injection in patients with Crohn‘s disease. Correlation with the therapeutic effectiveness of specific anti-inflammatory treatment. Eur J Radiol. 2009;69:438-444. [Cited in This Article: ] |
| 24. | Kunihiro K, Hata J, Manabe N, Mitsuoka Y, Tanaka S, Haruma K, Chayama K. Predicting the need for surgery in Crohn‘s disease with contrast harmonic ultrasound. Scand J Gastroenterol. 2007;42:577-585. [Cited in This Article: ] |
| 25. | Maconi G, Sampietro GM, Sartani A, Bianchi Porro G. Bowel ultrasound in Crohn‘s disease: surgical perspective. Int J Colorectal Dis. 2008;23:339-347. [Cited in This Article: ] |
| 26. | Maconi G, Parente F, Bollani S, Imbesi V, Ardizzone S, Russo A, Bianchi Porro G. Factors affecting splanchnic haemodynamics in Crohn‘s disease: a prospective controlled study using Doppler ultrasound. Gut. 1998;43:645-650. [Cited in This Article: ] |
| 27. | Ludwig D, Wiener S, Brüning A, Schwarting K, Jantschek G, Stange EF. Mesenteric blood flow is related to disease activity and risk of relapse in Crohn‘s disease: a prospective follow-up study. Am J Gastroenterol. 1999;94:2942-2950. [Cited in This Article: ] |
| 28. | Homann N, Klarmann U, Fellermann K, Brüning A, Klingenberg-Noftz R, Witthöft T, Stange EF, Ludwig D. Mesenteric pulsatility index analysis predicts response to azathioprine in patients with Crohn‘s disease. Inflamm Bowel Dis. 2005;11:126-132. [Cited in This Article: ] |
| 29. | Kumar P, Domjan J, Bhandari P, Ellis R, Higginson A. Is there an association between intestinal perfusion and Crohn‘s disease activity? A feasibility study using contrast-enhanced ultrasound. Br J Radiol. 2009;82:112-117. [Cited in This Article: ] |
| 30. | Maconi G, Greco S, Duca P, Ardizzone S, Massari A, Cassinotti A, Radice E, Porro GB. Prevalence and clinical significance of sonographic evidence of mesenteric fat alterations in Crohn‘s disease. Inflamm Bowel Dis. 2008;14:1555-1561. [Cited in This Article: ] |
| 31. | Maconi G, Bollani S, Bianchi Porro G. Ultrasonographic detection of intestinal complications in Crohn‘s disease. Dig Dis Sci. 1996;41:1643-1648. [Cited in This Article: ] |
| 32. | Maconi G, Di Sabatino A, Ardizzone S, Greco S, Colombo E, Russo A, Cassinotti A, Casini V, Corazza GR, Bianchi Porro G. Prevalence and clinical significance of sonographic detection of enlarged regional lymph nodes in Crohn‘s disease. Scand J Gastroenterol. 2005;40:1328-1333. [Cited in This Article: ] |
| 33. | Hollerweger A, Macheiner P, Dirks K, Dietrich CF. [Differential diagnosis of severe hypoechoic oedema of the small bowel]. Ultraschall Med. 2006;27:234-239. [Cited in This Article: ] |
| 34. | Maconi G, Sampietro GM, Russo A, Bollani S, Cristaldi M, Parente F, Dottorini F, Bianchi Porro G. The vascularity of internal fistulae in Crohn‘s disease: an in vivo power Doppler ultrasonography assessment. Gut. 2002;50:496-500. [Cited in This Article: ] |
| 35. | Seitz K, Reuss J. [Sonographic detection of fistulas in Crohn disease]. Ultraschall Med. 1986;7:281-283. [Cited in This Article: ] |
| 36. | Orsoni P, Barthet M, Portier F, Panuel M, Desjeux A, Grimaud JC. Prospective comparison of endosonography, magnetic resonance imaging and surgical findings in anorectal fistula and abscess complicating Crohn‘s disease. Br J Surg. 1999;86:360-364. [Cited in This Article: ] |
| 37. | Neye H, Ensberg D, Rauh P, Peitz U, Mönkemüller K, Treiber G, Klauck S, Malfertheiner P, Rickes S. Impact of high-resolution transabdominal ultrasound in the diagnosis of complications of Crohn‘s disease. Scand J Gastroenterol. 2010;45:690-695. [Cited in This Article: ] |
| 38. | Hurlstone DP, Sanders DS, Lobo AJ, McAlindon ME, Cross SS. Prospective evaluation of high-frequency mini-probe ultrasound colonoscopic imaging in ulcerative colitis: a valid tool for predicting clinical severity. Eur J Gastroenterol Hepatol. 2005;17:1325-1331. [Cited in This Article: ] |
| 39. | Stiatti A, Martinuzzi A, Bartolini M, Lascialfari L, Trallori G, Morettini A. [Ultrasonography in the diagnosis of chronic inflammatory intestinal disease]. Radiol Med. 1990;80:301-303. [Cited in This Article: ] |
| 40. | Dietrich CF. Significance of abdominal ultrasound in inflammatory bowel disease. Dig Dis. 2009;27:482-493. [Cited in This Article: ] |
| 41. | Pascu M, Roznowski AB, Müller HP, Adler A, Wiedenmann B, Dignass AU. Clinical relevance of transabdominal ultrasonography and magnetic resonance imaging in patients with inflammatory bowel disease of the terminal ileum and large bowel. Inflamm Bowel Dis. 2004;10:373-382. [Cited in This Article: ] |
| 42. | Parente F, Molteni M, Marino B, Colli A, Ardizzone S, Greco S, Sampietro G, Gallus S. Bowel ultrasound and mucosal healing in ulcerative colitis. Dig Dis. 2009;27:285-290. [Cited in This Article: ] |
| 43. | Parente F, Molteni M, Marino B, Colli A, Ardizzone S, Greco S, Sampietro G, Foschi D, Gallus S. Are colonoscopy and bowel ultrasound useful for assessing response to short-term therapy and predicting disease outcome of moderate-to-severe forms of ulcerative colitis?: a prospective study. Am J Gastroenterol. 2010;105:1150-1157. [Cited in This Article: ] |
| 44. | Ludwig D, Wiener S, Brüning A, Schwarting K, Jantschek G, Fellermann K, Stahl M, Stange EF. Mesenteric blood flow is related to disease activity and risk of relapse in ulcerative colitis: a prospective follow up study. Gut. 1999;45:546-552. [Cited in This Article: ] |
| 45. | Siğirci A, Baysal T, Kutlu R, Aladağ M, Saraç K, Harputluoğlu H. Doppler sonography of the inferior and superior mesenteric arteries in ulcerative colitis. J Clin Ultrasound. 2001;29:130-139. [Cited in This Article: ] |
| 46. | Yamaguchi T, Yoshida S, Tanaka S, Takemura Y, Oka S, Yoshihara M, Yamada H, Chayama K. Predicting the clinical response to cytapheresis in steroid-refractory or -dependent ulcerative colitis using contrast-enhanced ultrasonography. Scand J Gastroenterol. 2009;44:831-837. [Cited in This Article: ] |