INTRODUCTION
The pathogenesis of inflammatory bowel diseases (IBD) has not been completely resolved. However, the general hypothesis that in genetically predisposed people the exposure to distinct environmental factors results in a dysregulation of the mucosal immune system is still valid. Before genetics started to dominate the field, immunology identified various factors as critical in initiating intestinal inflammation. These studies included the functional characterization of T cell subpopulations and the relevance of the specific cytokines released. While the effector T cell response reflects one arm of the immune system, genetic work as well as studies on barrier function shed light on the second arm of the immune system. These two forms of immunity can be described as: (1) a more simplistic innate form, recognizing evolutionary conserved bacterial and viral patterns, resulting in a rapid but limited response; and (2) the adaptive form develops a highly specific immune response, that requires more time to evolve but provides immunological memory.
Each component plays a key role in the pathogenesis of IBD. Thus research in the field could be divided into groups focusing on the adaptive immune system, while other groups revealed innate factors at the site of epithelial cells or within the lamina propria which play a critical role. Cross-regulation of the innate and adaptive immune system was widely ignored.
Genome-wide association studies (GWAS) shed new light into the understanding of the pathogenesis[1,2]. The results obtained from GWAS not only confirmed the relevance of earlier characterized pathways, but also opened novel avenues and, in particular, provided strong evidence for a close link between the innate and the adaptive immune system in regulating the sensitive balance of the mucosal immune system.
Probably even more remarkable is the fact that GWAS have identified a large number of major loci, with many associations shared between various autoimmune diseases. These associations highlight key roles for lymphocyte activation, and prioritize specific cytokine pathways and mechanisms of host-microbe recognition[3]. Thus the close link and cross-regulation between the innate and adaptive immune system plays a critical role not only in IBD, but also in other autoimmune diseases. Interestingly, similarities of association patterns between various autoimmune diseases are particularly intriguing. Thus the question occurs as to which cells belong to the innate and which to the adaptive immune system.
In the intestine, innate immunity includes the epithelial barrier and phagocytic cells within the lamina propria (e.g. macrophages, dendritic cells, and neutrophils). Notably, patients exhibiting genetic defects in innate immunity (e.g. chronic granulomatous disease, Hermansky Pudlak syndrome) have an increase incidence of IBD[4,5]. This correlation has led to the development of agents used to boost innate immunity (e.g. granulocyte macrophage colony-stimulating factor) as therapeutic agents in IBD[6].
T lymphocytes represent the key cell population of the adaptive immunity arm. T cells become activated, secrete cytokines and affect all other cell types within a local environment (macrophages, dendritic cells, neutrophils, epithelium, endothelial cells, stromal elements). Both human and murine studies have led to the recognition that different T cell subpopulations are aberrantly activated in Crohn’s disease (CD) versus ulcerative colitis (UC)[7,8]. T helper cell type 1 (Th1)-mediated immune responses are typically evoked in response to an intracellular pathogen presented by an antigen-presenting cell in the presence of interleukin (IL)-12. The coordinated immune response is elicited to localize the infectious agent and to secrete factors that either promote apoptosis [e.g. interferon (IFN)-γ, tumor necrosis factor-α] or induce the differentiation of cytotoxic T lymphocytes. The hallmark of a Th1 response is granuloma[9]. Recently, an additional Th subset has been described, the so-called Th17 cells[10]. These cells produce IL-17 and IL-22, both of which are pro-inflammatory cytokines capable of promoting local tissue destruction. Th17 cells are activated by the combination of IL-6 and transforming growth factor (TGF)-β and are induced to further differentiate to mature IL-17-secreting cells by IL-23[11]. IL-23 belongs to the IL-12 family and shares the p40 subunit with IL-12. IL-17 as well as IL-22 are found at increased levels in inflamed CD mucosa suggesting that these cytokines play a role in disease pathogenesis[12,13]. Functional data from mice and men are in support of this hypothesis as described in more detail below. An additional Th subset consists of Th2 cells. These secrete IL-4, IL-5, and IL-13[14]. Th2 cells promote atopy with induction of IgE responses, and eosinophil and mast cell activation. UC was thought to represent a Th2-driven disease, but the absence of IL-4 in colonic tissue from UC patients and the observation that both IL-13 and IFN-γ are found at elevated levels in UC mucosa changed this dogma[15]. Recent data suggest that IL-13 originates from a natural killer T cell, and targets the epithelial cell to become dysfunctional[7,16]. Consequently, UC may be more of a superficial epithelial injury disorder.
Last, there are regulatory T cell subpopulations. This is of particular importance since the immunological defense of the intestine, in contrast to the systemic immune system, is one of suppression. Healthy individuals generally do not develop systemic immune responses against commensal flora or dietary antigens. Regulatory cells are responsible for the immunologically suppressed milieu in the intestinal mucosa. One group of regulatory cells contributing to this milieu are regulatory T cells[17,18]. Various subsets have been described as being involved in suppressing mucosal responses, but the detailed description is beyond the scope of this review. Tr1 secrete IL-10[19], a potent anti-inflammatory cytokine. The impact in intestinal inflammation is underlined by the fact that IL-10-deficient mice develop spontaneous intestinal inflammation[20]. Th3 cells produce TGF-β, another potent immunosuppressant cytokine that promotes IgA production while suppressing T and B cell activation[21]. In addition, there are CD4+ CD25+ Treg requiring the transcription factor FoxP3[17,18]. Remarkably, the absence of FoxP3 in humans (IPEX syndrome)[22,23] and mouse (Scurfy) is followed by an autoimmune endocrine disease, immunodeficiency, and an enteropathy mainly affecting the small bowel.
Although there are specific T cell subpopulations, exclusive activation of a single T cell subpopulation during the course of an immune response is impossible. There is additional experimental evidence suggesting that the classification of T cell subpopulations on the basis of cytokine secretion profiles are helpful but not absolute. It seems to be more important to underscore that a dysregulated immune response of any type is poorly tolerated by the gastrointestinal tracts and thus results in intestinal inflammation.
Under which conditions does dysregulation of innate and adaptive immunity occur? Luminal antigens cross the epithelial barrier, and this process is significantly increased during intestinal inflammation. The role of epithelial and Paneth cells within the first line of defense is discussed within this issue by others. The antigens reaching the lamina propria will first activate the innate immune system via pattern recognition receptors and second, be presented by professional antigen-presenting cells resulting in an effector T cell response as described above.
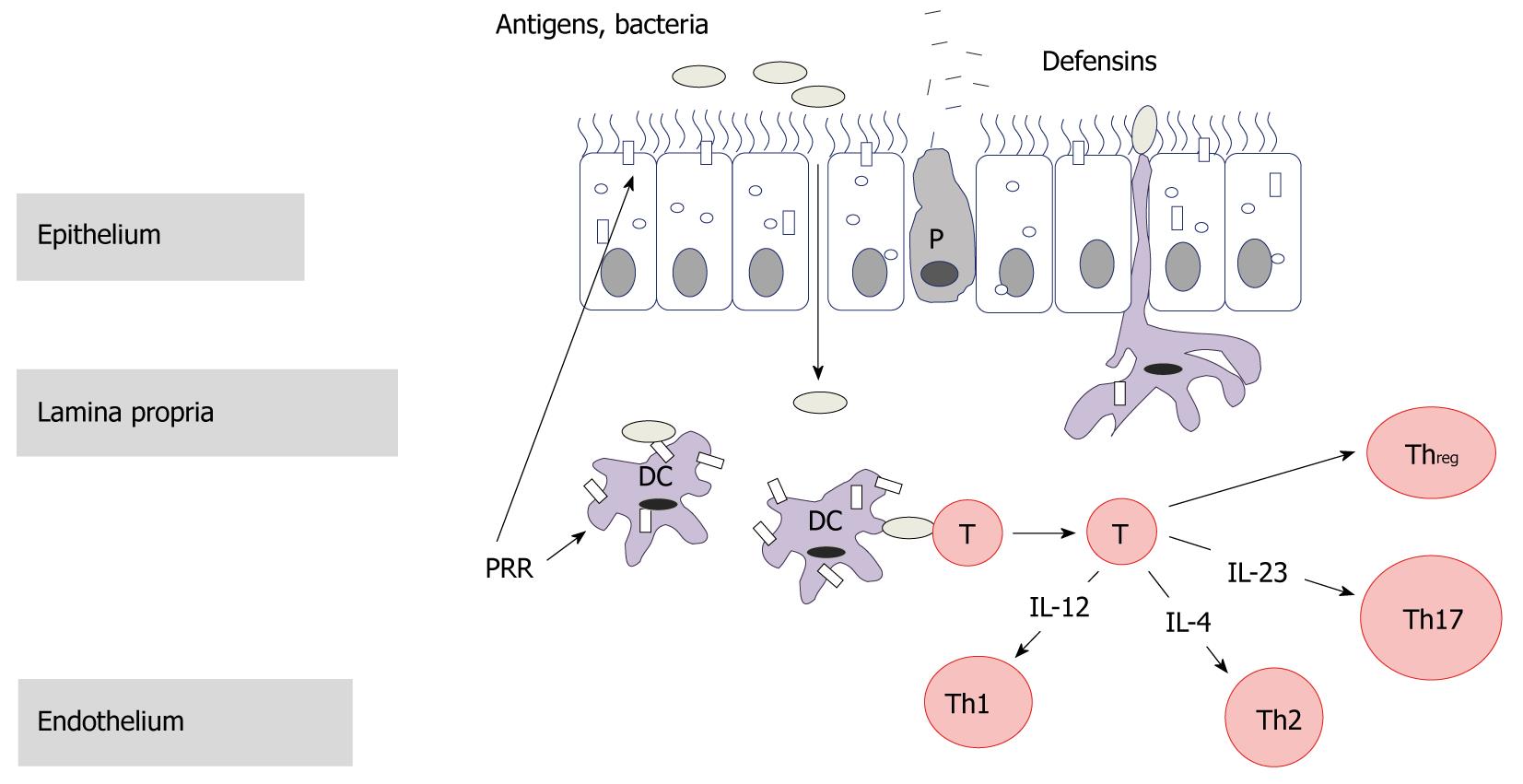
The present review will serve to illustrate the cross-regulation of the innate and adaptive immune system within this process and the impact on the pathogenesis of IBD. The cross-regulation will be described by selecting two examples. First, NOD2 a pattern recognition receptor, thus belonging primarily to the innate immune system. However, we will demonstrate that NOD2 exerts a direct impact on the regulation of the adaptive immune system. This cross-regulation leads to the second example that is directly linked to NOD2, namely the IL-12 family (for illustration see Figure 1).
Figure 1 Cell populations primarily belonging to the innate immune system are marked blue, and cells primarily categorized as cells of the adaptive immune system are marked red.
Purple marked cells identify the cross-link between both systems. IL: Interleukin.
NOD2
NOD2 (also designated CARD15) was identified by fine mapping of the IBD1 locus by positional cloning and candidate gene analysis as the first gene to be firmly associated with CD susceptibility in North American and European populations[24-26]. Three main variants, R702W, G908R, and 1007fs, exhibit the strongest CD association[27]. All three variants alter the C-terminal third of the gene product, and are within or close to a region of leucin-rich repeats thought to be involved in ligand recognition. Individuals with one of the three major disease-associated alleles have a 2-4-fold increased risk of developing CD, whereas homozygous or compound heterozygous carriers have a 15-40-fold increase in risk[28]. NOD2 is a cytoplasmic protein that serves as a microbial sensor for muramyl dipeptide (MDP), a peptidoglycan motif present in the cell wall of Gram-positive and Gram-negative bacteria[29,30]. There are currently three mechanistic explanations for the apparent inflammation-promoting functions of the variant NOD2 proteins in the literature. These hypotheses are not mutually exclusive and may remain valid in combination.
Transfection of wild-type, but not mutant NOD2 into intestinal epithelial cells inhibited uptake or growth of invasive bacteria[31]. Accordingly, mice lacking NOD2 showed a defect in intestinal innate defence against oral infection with L. monocytogenes, which was accompanied by diminished expression of at least two Paneth cell-derived antimicrobial peptides, Defcr4 and Decfcr-rs10[32]. CD patients with mutant NOD2 were shown to have decreased expression of the human Paneth cell α-defensins HD-5 and HD-6 in the small intestine[33], suggesting that CD-associated NOD2 mutations may be functionally equivalent to the loss of the protein in knockout mice. This model would initially restrict the function of NOD2 to the innate system, however when considering that a decrease in antimicrobial peptides results in an increase of bacterial translocation and thus activation of the adaptive immune system, the link becomes apparent.
Incubation of normal murine splenic macrophages with MDP resulted in suppression of IL-12p40 and IL-12p70 secretion induced by stimulation with Toll-like receptor (TLR) 2 ligands, such as peptidoglycan[34]. This suppression did not occur in cells lacking NOD2, or in cells expressing a mutant form of NOD2 after transfection. A similar mechanism appeared to act in vivo, as systemic administration of peptidoglycan to NOD2-/- mice induced more serum IL-12p40 and IL-12p70 compared to wild-type mice. The IL-12-enhancing effects occurred only via TLR2, but not other TLRs[34]. Thus TLR2 would be expected to be uniquely capable of promoting colonic inflammation under conditions of NOD2 deficiency. In this model NOD2 is activated as an innate receptor, however the effector response directly activates the adaptive immune system. This is further underlined by the findings described below for the IL-12 family.
Macrophages of mice homozygous for a mutant NOD2 allele (NOD2939iC) equivalent to the most common CD-associated allele (3020insC) were shown to secrete higher levels of the mature form of IL-1β and have elevated IL-1β mRNA levels, as well as increased IkB kinase and nuclear factor-κB activities, upon stimulation with MDP relative to wild-type macrophages but retain normal responses to TLR ligands[35]. Simultaneously, these mice exhibited greater colonic inflammation upon experimental challenge with dextran sulfate sodium, a phenotype that was attenuated by treatment with an IL-1 receptor antagonist[35]. These data suggest that the variant NOD2 protein expressed in these mice promotes processing of proIL-1β to mature, biologically active, IL-1β. Consistent with a role of IL-1β in regulating colitis, mice deficient in IL-1β converting enzyme have reduced inflammation in an experimental colitis model[36].
IL-12 FAMILY
A key role for the IL-12 family in the pathogenesis of IBD was initially suggested over a decade ago. In this study neutralizing antibodies targeting “IL-12” resulted in an amelioration of TNBS-induced colitis in mice[37]. In 2004, anti-IL-12 treatment showed efficacy in patients with CD, although the primary endpoint of this study was safety and not clinical efficacy[38]. In a more recent study investigating an anti-IL-12 antibody in patients with CD, the primary endpoint, namely the clinical response at week 8 was not achieved[39]. At this time, since IL-12 is the key Th1 cytokine, CD was classified as a Th1-mediated disease. These findings additionally supported the concept that CD is driven by the acquired immune system. In 2006, another member of the IL-12 family, namely IL-23, came into the focus in IBD. GWAS revealed a highly significant association between CD and the IL-23R gene on chromosome 1p31, which encodes a subunit of the IL-23 receptor. An uncommon coding variant confers strong protection against CD[40,41].
IL-12 and IL-23 share the subunit p40 that builds a heterodimer with p35, in the case of IL-12, and with the subunit p19, in the case of IL-23. IL-23 caused some confusion in the field, since the antibody administered in the clinical study published in 2004 targeted the IL-12 subunit p40, thus it suddenly became unclear whether the beneficial effect observed had been mediated by neutralizing IL-12 or IL-23. Several animal studies added valuable information to this controversy. In a model of CD40-induced colitis, neutralizing either the subunit p40 or the subunit p19 was followed by a significant reduction of the macroscopic and histologic inflammation score, indicating that in this model, IL-23 represents a critical pro-inflammatory mediator[42]. However, from this data one could not draw a conclusion on the relevance of IL-12. This question been answered in the following by applying the transfer model of colitis. In this model the disease-inducing population of naïve T cells isolated from wild-type mice is transferred to immunodeficient Rag-/- mice. Transfer of naïve T cells in either p40-/- or p19-/- Rag-/- mice failed to induce colitis, while no difference between the transfer of naïve T cells into Rag-/- versus Rag-/- p35-/- mice was observed. Consequently, at least in this model, IL-23 but not IL-12 represents the key pro-inflammatory cytokine[43]. However, selective neutralization of IL-23 may not always be beneficial. p19-/- mice are highly susceptible to T cell-mediated colitis induced by hapten. In the absence of IL-23, gut dendritic cells produced excessive amounts of IL-12 as a result of the missing regulatory effect of IL-23[44]. Thus, in the absence of IL-23, mice developed enhanced IL-12-driven mucosal immunopathology; whether such cross-regulation is relevant in patients with IBD remains to be determined.
The IL-12 family is closely linked to the innate immune system. IL-12 and IL-23 within the lamina propria are predominantly produced by macrophages and dendritic cells. Both cell populations can be activated via innate receptors on one hand and via the development of an adaptive immune response on the other hand. NOD2 is probably the best example for this central function. As described above, a mutation in NOD2 is followed by an increase in IL-12p70[34], thus directly linking a receptor of the innate immune system with the effector cascade of the adaptive immune system.