Published online Apr 14, 2011. doi: 10.3748/wjg.v17.i14.1848
Revised: June 9, 2010
Accepted: June 16, 2010
Published online: April 14, 2011
AIM: To investigate thrombotic microangiopathy (TMA) in liver transplantion, because TMA is an infrequent but life-threatening complication in the transplantation field.
METHODS: A total of 206 patients who underwent living-donor liver transplantation (LDLT) were evaluated, and the TMA-like disorder (TMALD) occurred in seven recipients.
RESULTS: These TMALD recipients showed poor outcomes in comparison with other 199 recipients. Although two TMALD recipients successfully recovered, the other five recipients finally died despite intensive treatments including repeated plasma exchange (PE) and re-transplantation. Histopathological analysis of liver biopsies after LDLT revealed obvious differences according to the outcomes. Qualitative analysis of antibodies against a disintegrin-like domain and metalloproteinase with thrombospondin type 1 motifs (ADAMTS-13) were negative in all patients. The fragmentation of red cells, the microhemorrhagic macules and the platelet counts were early markers for the suspicion of TMALD after LDLT. Although the absolute values of von Willebrand factor (vWF) and ADAMTS-13 did not necessarily reflect TMALD, the vWF/ADAMTS-13 ratio had a clear diagnostic value in all cases. The establishment of adequate treatments for TMALD, such as PE for ADAMTS-13 replenishment or treatments against inhibitory antibodies, must be decided according to each case.
CONCLUSION: The optimal induction of adequate therapies based on early recognition of TMALD by the reliable markers may confer a large advantage for TMALD after LDLT.
- Citation: Hori T, Kaido T, Oike F, Ogura Y, Ogawa K, Yonekawa Y, Hata K, Kawaguchi Y, Ueda M, Mori A, Segawa H, Yurugi K, Takada Y, Egawa H, Yoshizawa A, Kato T, Saito K, Wang L, Torii M, Chen F, Baine AMT, Gardner LB, Uemoto S. Thrombotic microangiopathy-like disorder after living-donor liver transplantation: A single-center experience in Japan. World J Gastroenterol 2011; 17(14): 1848-1857
- URL: https://www.wjgnet.com/1007-9327/full/v17/i14/1848.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i14.1848
Thrombotic microangiopathy (TMA) is a microvascular occlusive disorder induced by facilitation of endothelial damage and primary platelet (PLT) aggregation[1]. Currently, the concept of TMA encompasses two previous entries, i.e., thrombotic thrombocytopenic purpura and hemolytic uremic syndrome[2]. Although the specific pathophysiological mechanism is still not understood, many investigators have focused on a disintegrin-like domain and metalloproteinase with thrombospondin type 1 motifs (ADAMTS)-13 as a metalloproteinase that specially cleaves the multimeric von Willebrand factor (vWF)[3-5]. The vWF is an essential factor for the adhesion/aggregation of circulating PLTs associated with high shear stresses[6,7]. Following the identification of ADAMTS-13 in 2001[3-5], some investigators suggested that a deficiency of ADAMTS-13 activity[3,8] and/or inhibitory autoantibodies against ADAMTS-13[2,9] may cause TMA via a pathway in which unusually large vWF multimers elevate the shear stress and lead to excessive PLT clumping[6,7,10]. As a consequence, PLTs would be consumed in TMA patients, and finally result in organ failure because of microvascular occlusion.
Clinically, TMA patients develop thrombocytopenia accompanied by hemolytic anemia and show a hemorrhagic tendency[1,2,11]. The TMA is clinically defined as thrombocytopenia and microangiopathic hemolytic anemia with no apparent alternative explanations[1,12,13]. The diagnostic criteria for TMA are defined as follows[1,2,14,15]: (1) the presence of thrombocytopenia (PLT count < 5.0 × 104/mm3) or the progressive decline in PLT counts (decrease of > 3.0 × 104/mm3 within 24 h); (2) microangiopathic hemolytic anemia [hemoglobin (HB) < 8.0 g/dL]; (3) sharply elevated levels of serum lactate dehydrogenase (LDH) (typically > 500 IU/L); (4) the presence of fractionated erythrocytes in a blood smear; and (5) severe deficiency in ADAMTS-13 activity (< 5% in normal plasma) or prevalence of ADAMTS-13-specific antibodies (Abs) categorized as immunoglobulin G (IgG) isotypes.
TMA is well known as a fatal complication after transplantation, and its frequencies are documented to be 6% after bone marrow transplantation[16] and 3%-5% after solid-organ transplantation[17,18]. The first case of TMA in the liver transplantation (LT) field was reported in 1984[19], and thereafter some researchers have reported that the frequency of TMA after LT is 3.8%-5.0%[2,14]. Repeated plasma exchange (PE)/exchange transfusion (ET) is considered to be the standard therapy for TMA from the viewpoint of the replenishment of ADAMTS-13 and the depletion of inhibitory Abs[13,15,20-22]. To date, a total of 739 pediatric and 669 adult LTs have been performed in our institution. Although we have no experience of cases that completely fulfilled the TMA criteria described above, seven cases were retrospectively considered to be TMA-like disorder (TMALD) after living-donor liver transplantation (LDLT). Here, we focused on post-LDLT TMA cases and present our results for TMALD patients. The pathophysiological basis, the early marker for the recognition of TMALD, the reliable factors for diagnosis and further clinical strategies are discussed in the LDLT field.
A total of 155 adult and 51 pediatric recipients who underwent LDLT at Kyoto University Hospital from April 2006 to March 2009 were evaluated in this study. Seven patients (five adults and two pediatric recipients) showed thrombocytopenia and microangiopathic hemolytic anemia with no apparent alternative causes. They were given the intensive TMALD treatments.
These seven patients comprised one male and six females, and their age range was 0.8-65.2 years. The primary diseases for LDLT included two cases each of primary biliary cirrhosis and liver cirrhosis caused by hepatitis C virus (HCV), and one case each of liver cirrhosis caused by autoimmune hepatitis, biliary atresia (post-Kasai’s portoenterostomy) and fulminant hepatic failure (etiology unknown). The United Network for Organ Sharing statuses were estimated to be four cases of IIA, two of IIB and one of I. The mean Child-Pugh score was 12.0 ± 1.7 points (range, 10-14 points). The mean score of the model for end-stage liver disease (MELD) or pediatric end-stage liver disease (PELD) was 23.7 ± 8.8 points (range, 17-41 points). The ABO blood groups were characterized as three cases each of identical and incompatible and one case of compatible. The donor relationships were four spouses, one grandmother, one father and one son. The protocol of the study was approved by the Ethics Review Committee for Clinical Studies of Kyoto University Graduate School of Medicine.
There were three left-lobe grafts, two extended lateral-segment grafts and one right-lobe graft without the middle hepatic vein. The range of the graft/recipient weight ratios was 0.74-4.58. Normal findings for histopathological analyses of biopsy specimens during the donor operation were confirmed in all cases. The mean operative time was 690.7 ± 150.0 min (range, 480-873 min) and the mean blood loss was 4180.0 ± 2843.3 mL (range, 370-7700 mL). The mean cold ischemic time, warm ischemic time and anhepatic phase were 49.4 ± 18.9 min (range, 27-77 min), 63.0 ± 32.9 min (range, 40-133 min) and 150 ± 76.6 min (range, 51-267 min), respectively. All the recipients received blood transfusions of a red cell concentrate and fresh-frozen plasma (FFP) during LDLT, and two recipients received a PLT transfusion. The patient profiles are shown in Table 1.
| Recipients | ABO1 | TMALD2 | Clinical courses after TMALD | PE/ET3 | Additional surgery4 | Outcome |
| Pediatric | Incompatible | 21 | Herpes simplex sepsis | 0 | None | Alive |
| Pediatric | Incompatible | 6 | Pneumonitis | 4 | None | Alive |
| Adult | Identical | 14 | Hepatic encephalopathy, intraperitoneal bleeding | 9 | Surgical hemostasis (16) | Dead |
| Adult | Incompatible | 9 | Hepatic encephalopathy, brain edema gastroesophageal varices rupture, intrathoracic intraperitoneal, bleeding sepsis, aspergillus infection | 8 | Surgical hemostasis (21) Re-transplantation (22) | Dead |
| Adult | Compatible | 4 | Acute respiratory distress, syndrome intraperitoneal bleeding | 5 | None | Dead |
| Adult | Compatible | 10 | Intraperitoneal bleeding | 3 | Surgical hemostasis | Dead |
| Adult | Compatible | 14 | Hepatic arterial thrombosis | 10 | None | Dead |
Immunosuppression after LDLT was started with tacrolimus and methylprednisolone. The trough level of tacrolimus was maintained at 8-15 ng/mL during the early postoperative period, based on the clinical findings in each case. Calcineurin inhibitors (CNIs) were converted to cyclosporin A from tacrolimus at postoperative day (POD) 15 in one case. Methylprednisolone was given intravenously (1 mg/kg) once daily from POD 1 to POD 3 followed by 0.5 mg/kg once daily for the next three days. On POD 7, 0.3 mg/kg of methylprednisolone was given intravenously. Steroid administration was switched to oral prednisolone 0.3 mg/kg once daily on POD 8. This dose was reduced to 0.1 mg/kg at one month after LDLT. We had already overcome ABO-incompatibility in LDLT, and our regimens for these recipients, including heparin usage, were described previously[23,24].
To exclude other diseases, such as humoral rejection (HR), hematological diseases, heparin-induced thrombocytopenia and autoimmune diseases, detailed examinations, such as bone-marrow puncture, liver needle biopsy, immunological assays and measurements of anti-platelet factor 4/heparin Abs and anti-PLT autoantibodies were performed.
In our institution, laboratory examinations were routinely performed at least every 8-12 h in all recipients during the early postoperative period after LDLT and in critical LDLT recipients. The appearances of fragmentation of red cells (FRC) and microhemorrhagic macules (MHMs) were checked, and temporal changes in the counts or levels of PLTs, HB, LDH, prothrombin time-international normalized ratio (PT-INR) and total bilirubin (T-BIL) were estimated.
The values for vWF and ADAMTS-13 were measured by enzyme-linked immunosorbent assays, and subsequently used to calculate the vWF/ADAMTS-13 ratio. Quantitative determination of inhibitory IgG (anti-ADAMTS-13 Abs) was performed by the Bethesda method (the detection limit was 0.5 Bethesda units/mL).
Normal ranges of vWF, ADAMTS-13 and the vWF/ADAMTS-13 ratio were calculated in a control group, with 10% rejection region. A control group was composed of 54 healthy volunteers (26 males and 29 females). Normal values of vWF, ADAMTS-13 and the vWF/ADAMTS-13 ratio were 60%-170%, 70%-120%, and 0.51-2.43, respectively. The anti-ADAMTS-13 Abs were non-existent in healthy individuals (under the detection limit).
We still do not have any experience of patients who strictly fulfilled all of the TMA criteria. In this study, TMALD was defined as the LDLT recipients who fulfilled the diagnostic criteria of TMA without the absolute value of ADAMTS-13 activity or the presence of ADAMTS-13-specific Abs.
Previous investigators have documented the important factors for LT outcomes, such as recipient age, disease, donor age, MELD/PELD score, ABO incompatibility, lymphocyte cross-match, cold ischemic time, operative time, blood loss, graft-recipient weight ratio, the type/number of anastomosis and conventional liver function test at early postoperative period[23,25-33]. There were no statistical differences between the 2 groups in each risk factor for LDLT outcomes, respectively.
A protocol liver needle biopsy (LNB) is not employed in our institution. However, all cases underwent an LNB before and after the treatments for TMALD, except for one case in which an LNB could not be performed because of a severe hemorrhagic tendency. The graft damage score was estimated according to established guidelines[34], and was calculated as the total of points of the following parenchymal features: hepatocyte ballooning (0 point = no, 1 = yes), hepatocyte necrosis (0 = none, 1 = small foci, 2 = confluent areas, 3 = bridging necrosis), congestion (0 = no, 1 = yes), microvesicular fat (0 = none, 1 = 1/3 hepatocytes, 2 = between 1/3 and 2/3 hepatocytes, 3 = 2/3 hepatocytes), neutrophil aggregate (0 = none, 1 = minimal, 2 = moderate, 3 = extensive), and cholestasis (0 = none, 1 = mild, 2 = moderate, 3 = severe).
The results are expressed as the mean ± SD. The differences between unpaired continuous or discontinuous data between two groups were analyzed by Student’s t-test. Survival rates were calculated by the Kaplan-Meier method, and the log-rank test was used for between-group comparisons. All calculations were performed using SPSS Software Version 16.0 (SPSS Inc., Chicago, IL 60606, USA). Differences with P values of < 0.05 were considered to be statistically significant.
The protocol of this study was approved by the Ethics Review Committee for Clinical Studies of Kyoto University Graduate School of Medicine.
TMALD was confirmed in seven cases, and the frequency in our institution was 3.4%. The definite diagnoses were reached at a mean time point of POD 11.1 ± 5.7 (range, 4-21 d).
Five adult recipients clearly showed prolonged jaundice after TMALD [T-BIL peak, 38.9 ± 11.2 mg/dL (range, 21.0-51.0 mg/dL)]. Four of these five cases suffered massive bleeding because of a hemorrhagic tendency in TMALD, and surgical hemostasis was emergently required in three cases. Thrombosis in the hepatic blood flow occurred after TMALD in one of the five cases. Consequently, these five cases fell into graft loss after TMALD, and two of the five recipients suffered hepatic encephalopathy. The details of the clinical courses are shown in Table 1.
On the other hand, two pediatric recipients (ABO-incompatible combinations) showed good responses to the treatments for TMALD, and recovered from their severe graft damage (Table 1).
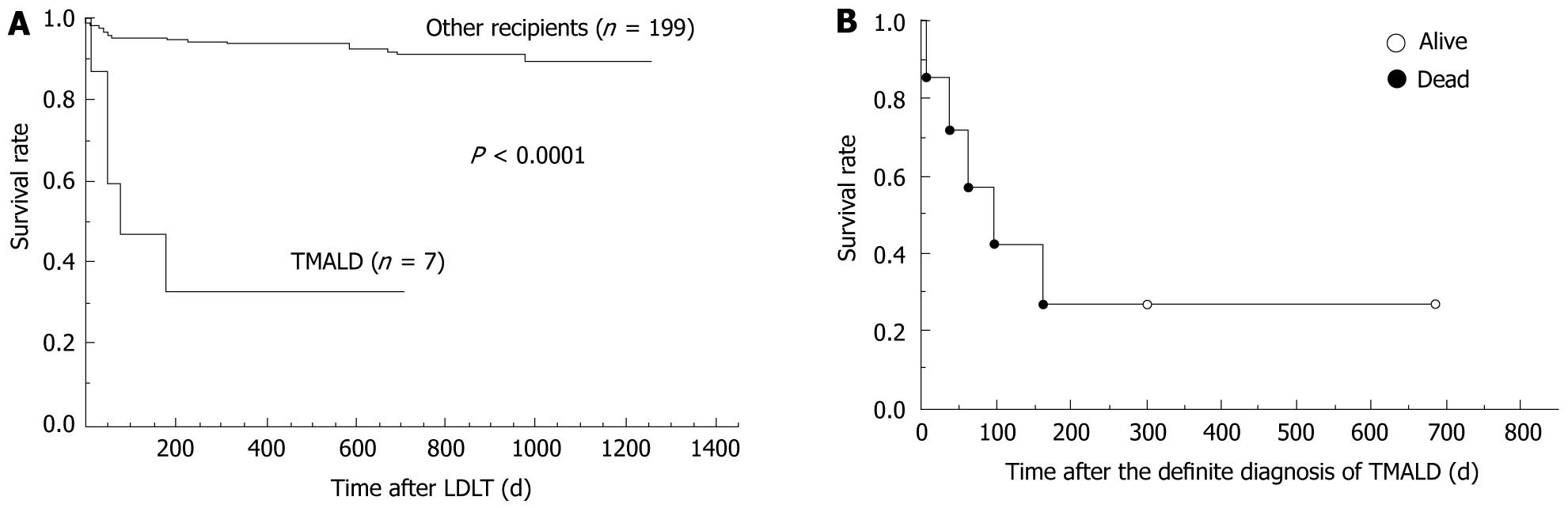
In comparisons of the survival rates, the TMALD patients clearly showed poor outcomes compared with the other patients who underwent LDLT during the same period in our institution (Figure 1A).
Critical events occurred after TMALD in five recipients and their clinical conditions tended to become worse despite the intensive treatments (Table 1). All of these five recipients with TMALD finally died despite intensive treatments (Figure 1B), and their mean survival time after the TMALD diagnosis was only 70 ± 63 d (range, 5-161 d). Although one of the five patients underwent a re-transplantation because of graft loss, she also finally died (Table 1).
The fragmentation of red cells was obviously detected in all cases. The haptoglobin level was also decreased to < 5.0 mg/dL in all cases. MHMs were observed in five patients. The mean lowest value of the PLT counts was 1.2 ± 5.5 × 104/mm3 (range, 0.6-2.1 × 104/mm3) while that of HB was 6.61 ± 0.68 g/dL (range, 5.9-7.6 g/dL). The mean peak value of the LDH level was 1202.3 ± 603.9 IU/L (range, 518-2089 IU/L). The PT-INR value was prolonged to > 1.5 in only one case at the time of a definite diagnosis, although the mean value for the most prolonged PT-INR after TMALD was 2.66 ± 1.85 (range, 1.22-5.64).
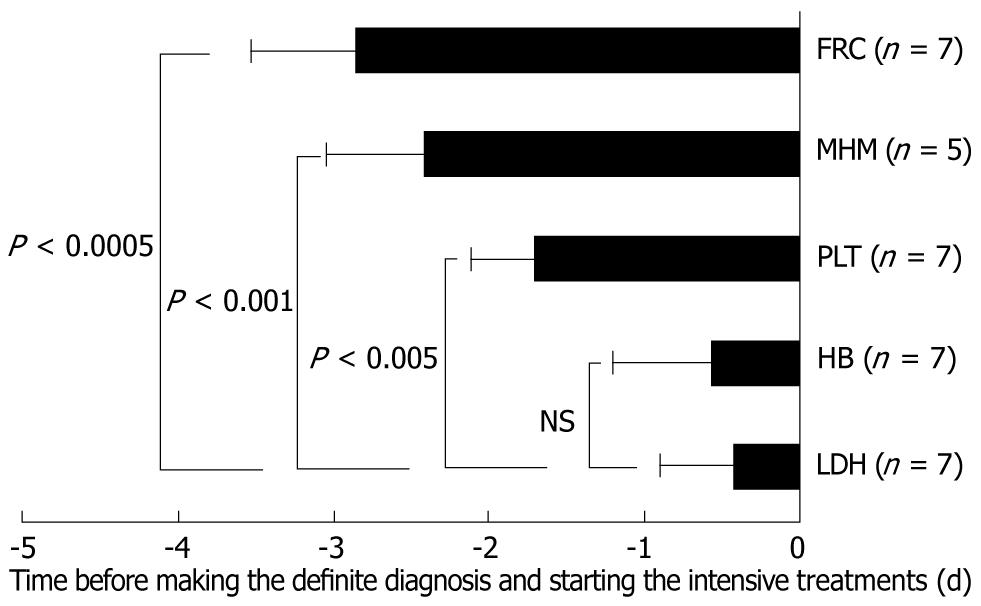
In two cases, the PLT counts before TMA onset were continuously < 5.0 × 104/mm3. Progressive decreases of > 3.0 × 104/mm3 within 24 h were considered to be the time points of fulfilling the TMALD criteria in these cases. Although MHMs were not necessarily observed in all cases, MHMs surprisingly appeared at the early phase of TMALD onset after LDLT, as well as FCR (Figure 2). In each case, elevated LDH levels proved decisive for making a diagnosis of TMALD, although their appearances were mostly late, similar to the case for HB (Figure 2).
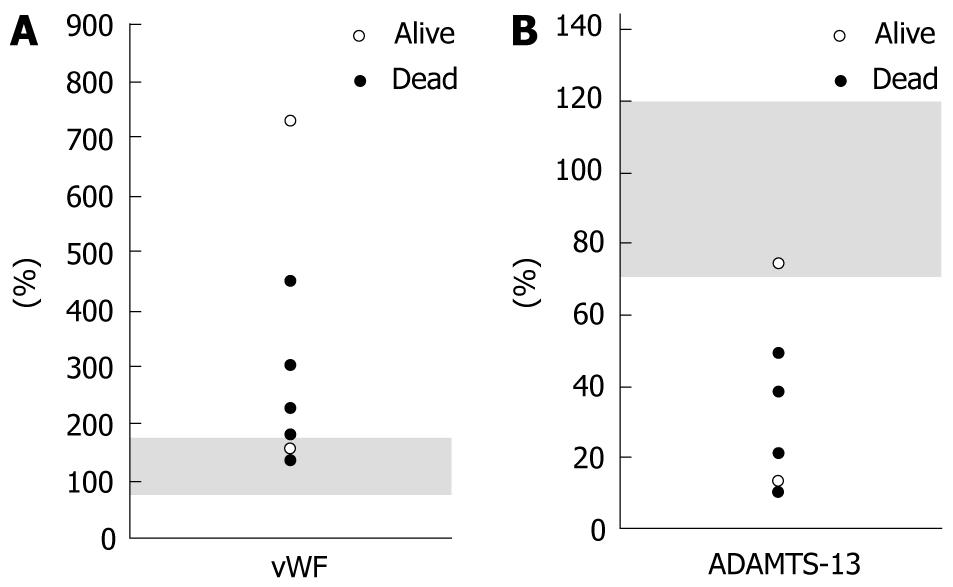
In two cases, the vWF levels were within the normal range. The mean absolute value of vWF was 306.6% ± 212.0% (range, 135%-723%) (Figure 3A). The ADAMTS-13 level also revealed no abnormalities in one case. The mean absolute value of ADAMTS-13 was 31.1% ± 24.3% (range, 10%-75%) (Figure 3B). Therefore, the degrees of alterations in these absolute values did not seem to precisely reflect the TMALD outcomes after LDLT (Figure 3).
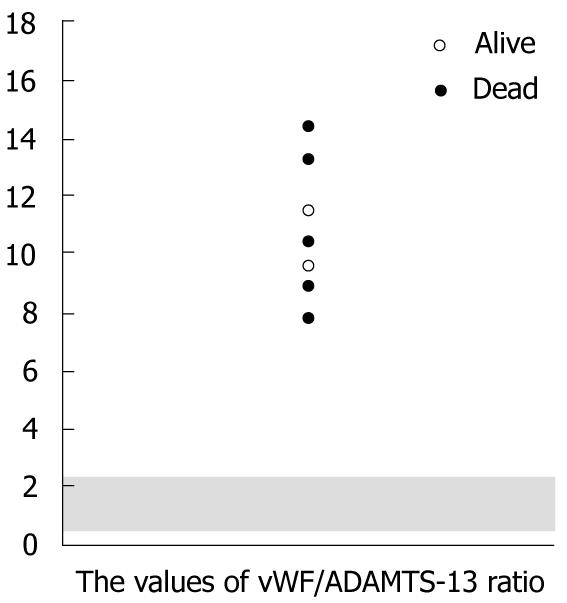
On the contrary, from the viewpoint of an imbalance between vWF and ADAMTS-13, the ratio of vWF/ADAMTS-13 strictly revealed the abnormalities in all of the TMALD recipients after LDLT (Figure 4). The mean value for the vWF/ADAMTS-13 ratio was 11.0 ± 2.4 (range, 7.8-14.6).
Qualitative analysis of anti-ADAMTS-13 Abs produced negative results in all cases. Even in the quantitative determinations, only two cases showed subtle elevations at approximately the cut-off level, although some TMA patients in the hematological field in our hospital showed obvious elevation of anti-ADAMTS-13 Abs.
Intensive care procedures for secondary complications, such as continuous hemodiafiltration for renal failure, respiratory control for pulmonary dysfunction and intravenous administration of antibiotics for sepsis, were performed in each case based on real-time estimations of their physical conditions, if necessary.
PE/ET (FFP 80-100 mL/kg per day) were repeated as the standard therapy for TMALD in six cases immediately after making a definite diagnosis of TMALD. The mean number of times of repeated PE/ET was 6.5 ± 2.9 (range, 3-10). Repeated PE therapy was not introduced in only one patient who successfully recovered from TMALD. It has been suggested since that repeated PE for the liver failure before LDLT can cause a recurrence of herpes simplex infection after LDLT because of the depletion of antigen-specific Abs by PE and the non-specific immunosuppression after LDLT.
Histopathological analysis of LNB specimens clearly revealed that the five recipients who had poor outcomes suffered graft loss after TMALD.
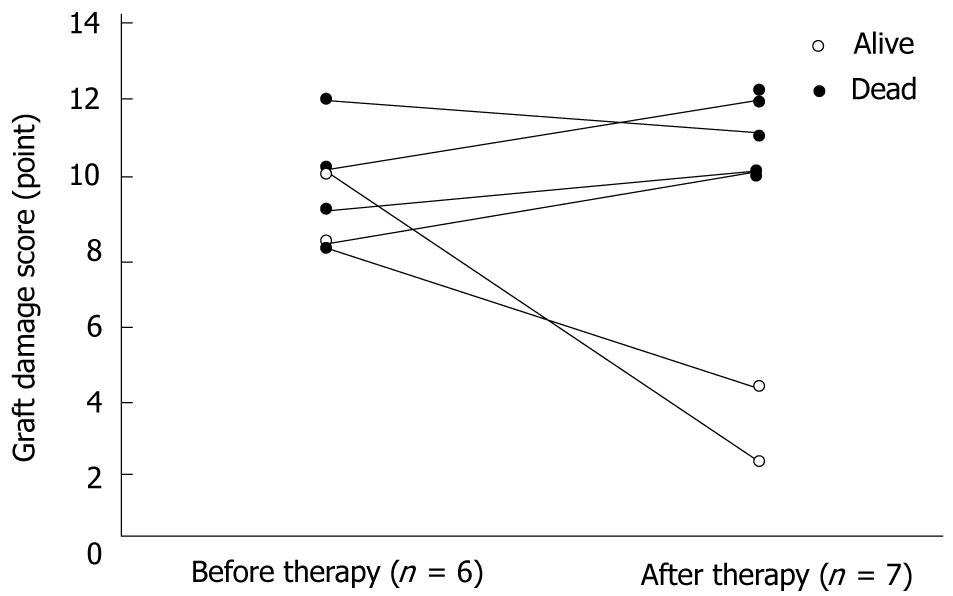
The mean graft damage scores before and after TMALD treatments in all patients were 8.9 ± 2.2 points (range, 5-12 points) and 8.7 ± 4.0 points (range, 2-12 points), respectively (Figure 5).
In comparisons of the histopathological findings before and after TMALD treatments, the results clearly demonstrated that the five recipients who had poor outcomes still showed severe graft parenchymal damage despite of repeated PE [mean graft damage score after TMALD treatments, 8.8 ± 2.6 points (range, 5-12 points)], although the two surviving recipients recovered from their graft parenchymal damage after repeated ET (four times) or no PE/ET treatment. The graft damage scores after TMALD treatments in the successfully treated recipients decreased to two or four points.
Although the specific mechanism remains unknown, previous reports have documented that CNIs and infections, including cytomegalovirus (CMV) and HCV infections, may be possible causes of TMA after LT[2,17,35-39]. Despite the fact that our institution is a major transplant center in Japan, the frequency of TMA in our institution may be slightly lower than those in previous reports[16,18]. A possible explanation for this is the well-controlled conditions aimed at preventing CMV and HCV infections after LDLT in our institution[40,41]. In particular, our clinical strategy brings about excellent long-term outcomes after LDLT for recipients with HCV[40], and the frequency of TMALD in such recipients is 3.2% (2 TMALD cases among 62 HCV patients after LDLT) in our institution. Paradoxically, uncontrolled HCV infection may be a trigger of TMA onset as previously described[2,42]. TMA occurs as a long-term complication after LT by association with viral infections[2], whereas our patients presented with TMALD during the early postoperative period. One of the possible explanations is the good control of viral infections as described above, although we are sometimes troubled by Epstein-Barr virus infections, including long-term complications of post-transplantation lymphoproliferative disorders[43]. Regarding CNIs, our results that CNIs were used in all cases (tacrolimus was switched to cyclosporin A in 1 case) reveal an undeniable possibility of CNI effects, as previously documented[17,35,36,39]. We also cannot negate the possibility that CNI introduction caused TMALD during the early postoperative period in our cases. However, we consider that complete CNI-free LDLT for preventing TMA is a distant ideal, since CNIs are key immunosuppressants for LDLT during this period[15,44].
The mortality of TMA patients without effective treatments can be as high as 90%[1,16], and 10%-20% of TMA patients still die even after PE/ET[13,45]. The outcomes of TMALD patients are truly poor in our institution. Serious problems, such as massive bleeding, vascular thrombosis and organ failures, actually appeared in these recipients because of PLT consumption and microvascular occlusion mediated by TMALD. Five TMALD patients died without long survival times despite receiving continuous intensive treatments including re-transplantation for graft loss. Our own results indicate that further improvements in clinical strategies, including diagnostic methods and effective treatments based on the TMA mechanism after LDLT, are still needed.
Currently, the criteria for TMA are established as described above. We still do not have any experience of patients who strictly fulfilled all of the TMA criteria. Therefore, we consider that our patients were TMALD rather than TMA. However, we think that our recipients are homogeneously the same as other TMA patients in their clinical events. Based on our results in assays for TMA diagnosis, TMA recipients after LT may not be considered in the same manner as congenital or autoimmune TMA patients purely in the hematological field, when making a definite diagnosis. Inherently, TMA involves microvascular occlusion and unusually large vWF multimers are present at its inception[1,7]. Although we initially focused on vWF and ADAMTS-13, similar to previous studies[2-5,8,9,14], we found that these absolute values were not necessarily reliable for the confirmation of TMA in LDLT recipients. Our results revealed only tendencies toward high vWF and low ADAMTS-13, while a few cases showed normal absolute values. However, the balance between vWF and ADAMTS-13 was clearly broken down. We suggest that this imbalance is a true entity in TMA after LDLT, and that evaluation of the vWF/ADAMTS-13 ratio is more suitable after LDLT to establish the patient’s condition and achieve a precise diagnosis of TMA. Since a precise diagnosis is always required in order to administer appropriate treatments[2,14,15], we suggest that this variable is one of the keys for improving the TMALD outcomes after LDLT.
Immunologically and pharmaceutically, numerous unpreventable influences exist in LDLT recipients. The phenomena of microvascular occlusive disorders accompanied by endothelial damage, PLT aggregation and hemolysis will be observed for other reasons, such as HR. On making a diagnosis of TMA after LDLT, many examinations including hematological, immunological and histopathological assays are therefore required to rule out other possible reasons, such as HR, sepsis and autoantibodies as well as HIT and drug-induced disorders[2,14]. Therefore, reaching a definite diagnosis of TMA usually requires many considerations and much time. Actually, in our results, we retrospectively considered that TMALD onsets were earlier rather than at the point of diagnosis and/or treatment induction. We suggest that although the LDH level has a specific sensitivity for TMA/TMALD this variable will not work as an early marker for TMA/TMALD. Decreases in PLT counts can cause serious problems which sometimes require additional surgery. FRC and PLT decreases were observed at earlier points, and unexpectedly, MHMs were also reliable as an early sign of TMALD after LDLT if they appeared. A possible explanation is the time-lag between an initial high shear stress and the resultant microvascular occlusion[6,7]. Ironically, transplant surgeons cannot decide actual treatments based on FRC, MHMs and PLT decreases even with earlier appearances, while TMA basically contraindicates a PLT transfusion. Although the changes over time in vWF and ADAMTS-13 could not be estimated in these patients because of cost-saving reasons, we hypothesize that the ratio of vWF/ADAMTS-13 is the earliest marker after LDLT for ruling out TMA and deciding appropriate treatments from the viewpoint of an essential cause of TMA.
Basically, the treatments must be chosen based on each cause of TMA, i.e. the lack of ADAMTS-13 and/or the inhibition of ADAMTS-13 via specific Abs[2,3,8,9]. We suggest that adequate therapies, i.e. PE/ET for ADAMTS-13 replenishment or additional treatments against inhibitory Abs, must be decided according to each cause of TMA/TMALD. The PE/ET has effects on both replenishment of ADAMTS-13 and depletion of inhibitory Abs against ADAMTS-13. Therefore, PE/ET (80-100 mL/kg per day) is widely considered as the standard therapy for TMA[13,15,20-22]. As described above, the essence of TMA after LDLT is an imbalance between vWF and ADAMTS-13, and we expect that PE/ET will have a sufficient effect from the viewpoint of the replenishment of ADAMTS-13. However, the five TMALD patients who finally died revealed that the graft loss caused by TMALD resulted in poor outcomes, even in a re-transplanted case, and that graft parenchymal damage became worse one-sidedly despite the intensive treatments. On the contrary, the other two TMALD patients were successfully treated, even though the treatment in one case excluded PE/ET. The graft damage caused by TMALD exhibited sufficient responses to the administered treatments in these successfully treated patients. Our results indicate that further improvements for TMALD after LDLT are truly required. We suggest that PE/ET itself has an anticipated efficacy in TMALD patients who lack ADAMTS-13[3,8]. Furthermore, optimal timing of the PE/ET induction according to the TMALD onset can improve the outcomes in these patients, even though the same treatment is performed[2,13-15,45]. The morbidity and mortality may increase if we hesitate to introduce the therapy until the criteria for TMA are completely fulfilled. We therefore suggest that earlier induction will improve the outcomes in ADAMTS-13-deficient patients when TMALD is suspected after LDLT based on early markers or an imbalance of the vWF/ADAMTS-13 ratio. Since our results showed that successful treatments are difficult once the TMA criteria are completely fulfilled, we consider that some data and/or signs accompanied by a PLT decrease are sufficient for PE/ET induction after LDLT, e.g. FCR, MHMs and a discrepancy of LDH elevation (even under the criteria) compared with other transaminases. On the other hand, PE/ET also has a benefit in TMALD patients accompanied by inhibitors from the viewpoint of Ab depletion[2,9,15]. However, our results for the TMALD patients who died might sustain the previous opinion that PE/ET has a limited usage for the depletion of specific Abs[15,46], and the actual results of poor outcomes oblige us to challenge some other therapies for TMALD patients after LDLT. Although we have no experience of Ab-positive TMA after LDLT, we now take anti-CD20 monoclonal Ab or intravenous high-dose IgG administration under consideration, if repeated PE/ET has insufficient effects on TMA progress.
Previous investigators suggested the diagnostic and therapeutic advices for TMA, and previous reports which focused TMA after LT were summarized in Table 2[2,14,15,37-39,47,48]. In our institution, the outcomes of TMALD recipients were poor despite the intensive treatments, especially in adult LDLT recipients. Previous researchers investigated the risk factors for TMA after LT[15,39], and Nishi et al[15] suggested that the onset POD, the value of urea nitrogen and the level of albumin were important for TMA outcomes after LDLT. In our institution, the levels of urea nitrogen were not measured routinely. A possible explanation for the poor outcomes of TMALD after LDLT in our institution was the early postoperative onset of TMALD within 30 d after LDLT (Table 1), though the albumin levels showed no statistical differences between groups. Current therapeutic strategies were still not enough, and we therefore speculated that an earlier induction of adequate treatments based on the early markers may improve the prognosis of TMALD after LDLT. We considered that the earlier recognition of TMALD and the earlier induction of treatments even under a suspicion of TMALD are crucial for LDLT recipients with TMALD.
| Year | The firstauthor | Patientnumber | PEtherapy | Focus | Diagnostic advices | Therapeutic advices |
| 2008 | Oya | 1 | + | The risk factor TMA, the relation of TMA and ABO compatibility | - | Reduction of CNI PE Intravenous infusion of high-dose γ-globulin |
| 2007 | Miyata | 4 | + | Plasminogen activator inhibitor type 1, the relation of TMA and ABO compatibility, early diagnosis of TMA for appropriate therapy | Plasminogen activator inhibitor type 1 the vWF value | Early recognition of TMA |
| 2007 | Akamatsu | 1 | + | The reation of TMA and CNI | - | CNI conversion |
| 2006 | Nishi | 18 | + | Poor outcome of TMA recipients PE therapy for TMA, the risk factors of TMA after LDLT | - | PE was not sufficient |
| 2005 | Banno | 5 | + | The analyzer of red blood cell fragmentation | Red blood cell fragmentation | - |
| 2005 | Taura | 10 | + | The relation of TMA and HCV infection | PE CNI conversion | |
| 2003 | Nakazawa | 1 | + | The vWF-cleaving protease, the inhibitors against this protease | The vWF-cleaving protease, the inhibitors against this protease | PE |
| 2003 | Ramasubbu | 1 | + | The relation of TMA and CMV | - | - |
TMA/TMALD stands as an infrequent but life-threatening complication in the LT field. Although retransplantation may seem to be a therapeutic option, this is strictly limited from the viewpoints of donor shortage and donor safety. We suggest that adequate therapies, i.e. PE/ET for ADAMTS-13 replenishment or additional treatments against inhibitory Abs, must be decided according to each cause of TMA/TMALD. We conclude that the optimal induction of these therapies based on earlier or reliable markers and the establishment of more advanced therapeutic strategies confers a large advantage for TMA/TMALD patients after LDLT and consequently improves LT outcomes.
The thrombotic microangiopathy (TMA) is an infrequent but life-threatening complication in the transplantation field. Here, the authors focused on post-living-donor liver transplantation (LDLT) TMA cases.
Some researchers have reported that the frequency of TMA after LT is 3.8%-5.0%. To date, a total of 1408 LTs have been performed in the authors’ institution. However, although the authors have no experience of cases that completely fulfilled the TMA criteria, seven cases were retrospectively considered to be thrombotic microangiopathy like disorder (TMALD) after LDLT. The pathophysiological basis, the early marker for the recognition of TMALD, the reliable factors for diagnosis and further clinical strategies are discussed in the LDLT field.
Only tendencies toward high von Willebrand factor (vWF) and low a disintegrin-like domain and metalloproteinase with thrombospondin type 1 motifs (ADAMTS)-13 were seen, while a few cases showed normal absolute values. However, the balance between vWF and ADAMTS-13 was clearly broken down. This balance reflected the condition of TMA after LDLT. Moreover, this variable is reliable along with earlier markers for TMALD recognition.
On making a diagnosis of TMA after LDLT, many examinations are required to rule out other possible reasons. Therefore, reaching a definite diagnosis of TMA usually requires many considerations and much time. The authors suggest that although the LDH level has a specific sensitivity for TMA/TMALD, this variable will not work as an early marker for TMA/TMALD. Fragmentation of red cell (FRC) and primary platelet (PLT) decreases were observed at earlier points and unexpectedly, microhemorrhagic macules were also reliable as an early sign of TMALD after LDLT if they appeared. Ironically, transplant surgeons cannot decide actual treatments based on FRC, MHMs and PLT decreases even with earlier appearances, while TMA basically contraindicates a PLT transfusion. They suggested that the ratio of vWF/ADAMTS-13 is the earliest marker after LDLT for ruling out TMA and deciding appropriate treatments from the viewpoint of an essential cause of TMA.
The establishment of adequate treatments for TMALD, such as plasma exchange for ADAMTS-13 replenishment or treatments against inhibitory antibodies, must be decided according to each case. The optimal induction of these therapies based on early recognition of TMALD by the early and/or reliable markers may confer a large advantage for TMALD recipients after LDLT. Consequently, their outcomes will be improved.
A very interesting manuscript for a rare disorder. It should be accepted with minor revisions.
Peer reviewer: R Mark Ghobrial, MD, PhD, FACS, FRCS (Ed), Director, Liver Center, Chief, Liver Transplantation Surgery, Director, Immunobiology Research Center, The Methodist Hospital, 6550 Fannin Street, SM1661, Houston, TX 77030, United States
S- Editor Wang YR L- Editor O’Neill M E- Editor Ma WH
| 1. | Moake JL. Thrombotic microangiopathies. N Engl J Med. 2002;347:589-600. [Cited in This Article: ] |
| 2. | Tamura S, Sugawara Y, Matsui Y, Kishi Y, Akamatsu N, Kaneko J, Makuuchi M. Thrombotic microangiopathy in living-donor liver transplantation. Transplantation. 2005;80:169-175. [Cited in This Article: ] |
| 3. | Levy GG, Nichols WC, Lian EC, Foroud T, McClintick JN, McGee BM, Yang AY, Siemieniak DR, Stark KR, Gruppo R. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413:488-494. [Cited in This Article: ] |
| 4. | Fujikawa K, Suzuki H, McMullen B, Chung D. Purification of human von Willebrand factor-cleaving protease and its identification as a new member of the metalloproteinase family. Blood. 2001;98:1662-1666. [Cited in This Article: ] |
| 5. | Gerritsen HE, Robles R, Lämmle B, Furlan M. Partial amino acid sequence of purified von Willebrand factor-cleaving protease. Blood. 2001;98:1654-1661. [Cited in This Article: ] |
| 6. | Savage B, Ginsberg MH, Ruggeri ZM. Influence of fibrillar collagen structure on the mechanisms of platelet thrombus formation under flow. Blood. 1999;94:2704-2715. [Cited in This Article: ] |
| 7. | Ruggeri ZM. Structure and function of von Willebrand factor. Thromb Haemost. 1999;82:576-584. [Cited in This Article: ] |
| 8. | Kokame K, Matsumoto M, Soejima K, Yagi H, Ishizashi H, Funato M, Tamai H, Konno M, Kamide K, Kawano Y. Mutations and common polymorphisms in ADAMTS13 gene responsible for von Willebrand factor-cleaving protease activity. Proc Natl Acad Sci USA. 2002;99:11902-11907. [Cited in This Article: ] |
| 9. | Soejima K, Mimura N, Hirashima M, Maeda H, Hamamoto T, Nakagaki T, Nozaki C. A novel human metalloprotease synthesized in the liver and secreted into the blood: possibly, the von Willebrand factor-cleaving protease? J Biochem. 2001;130:475-480. [Cited in This Article: ] |
| 10. | Kokame K, Nobe Y, Kokubo Y, Okayama A, Miyata T. FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br J Haematol. 2005;129:93-100. [Cited in This Article: ] |
| 11. | George JN. Tibor Greenwalt Award. The Oklahoma Thrombotic Thrombocytopenic Purpura-Hemolytic Uremic Syndrome Registry: a program for patient care, education and research. Transfusion. 2004;44:1384-1392. [Cited in This Article: ] |
| 12. | George JN. How I treat patients with thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Blood. 2000;96:1223-1229. [Cited in This Article: ] |
| 13. | Rock GA, Shumak KH, Buskard NA, Blanchette VS, Kelton JG, Nair RC, Spasoff RA. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian Apheresis Study Group. N Engl J Med. 1991;325:393-397. [Cited in This Article: ] |
| 14. | Miyata R, Shimazu M, Tanabe M, Kawachi S, Hoshino K, Wakabayashi G, Kawai Y, Kitajima M. Clinical characteristics of thrombotic microangiopathy following ABO incompatible living donor liver transplantation. Liver Transpl. 2007;13:1455-1462. [Cited in This Article: ] |
| 15. | Nishi H, Hanafusa N, Kondo Y, Nangaku M, Sugawara Y, Makuuchi M, Noiri E, Fujita T. Clinical outcome of thrombotic microangiopathy after living-donor liver transplantation treated with plasma exchange therapy. Clin J Am Soc Nephrol. 2006;1:811-819. [Cited in This Article: ] |
| 16. | Pettitt AR, Clark RE. Thrombotic microangiopathy following bone marrow transplantation. Bone Marrow Transplant. 1994;14:495-504. [Cited in This Article: ] |
| 17. | Trimarchi HM, Truong LD, Brennan S, Gonzalez JM, Suki WN. FK506-associated thrombotic microangiopathy: report of two cases and review of the literature. Transplantation. 1999;67:539-544. [Cited in This Article: ] |
| 18. | Humar A, Jessurun J, Sharp HL, Gruessner RW. Hemolytic uremic syndrome in small-bowel transplant recipients: the first two case reports. Transpl Int. 1999;12:387-390. [Cited in This Article: ] |
| 19. | Bonser RS, Adu D, Franklin I, McMaster P. Cyclosporin-induced haemolytic uraemic syndrome in liver allograft recipient. Lancet. 1984;2:1337. [Cited in This Article: ] |
| 20. | Ulinski T, Charpentier A, Colombat M, Desconclois C, Mougenot B, Fremaux-Bacchi V, Suberbielle C, Deschênes G, Bensman A, Veyradier A. From humoral rejection to generalized thrombotic microangiopathy--role of acquired ADAMTS13 deficiency in a renal allograft recipient. Am J Transplant. 2006;6:3030-3036. [Cited in This Article: ] |
| 21. | Llamas P, Romero R, Cabrera R, Sanjuán I, Forés R, Fernández MN. Management of thrombotic microangiopathy following allogeneic transplantation: what is the role of plasma exchange? Bone Marrow Transplant. 1997;20:305-306. [Cited in This Article: ] |
| 22. | Ferrari S, Scheiflinger F, Rieger M, Mudde G, Wolf M, Coppo P, Girma JP, Azoulay E, Brun-Buisson C, Fakhouri F. Prognostic value of anti-ADAMTS 13 antibody features (Ig isotype, titer, and inhibitory effect) in a cohort of 35 adult French patients undergoing a first episode of thrombotic microangiopathy with undetectable ADAMTS 13 activity. Blood. 2007;109:2815-2822. [Cited in This Article: ] |
| 23. | Egawa H, Teramukai S, Haga H, Tanabe M, Fukushima M, Shimazu M. Present status of ABO-incompatible living donor liver transplantation in Japan. Hepatology. 2008;47:143-152. [Cited in This Article: ] |
| 24. | Egawa H, Ohmori K, Haga H, Tsuji H, Yurugi K, Miyagawa-Hayashino A, Oike F, Fukuda A, Yoshizawa J, Takada Y. B-cell surface marker analysis for improvement of rituximab prophylaxis in ABO-incompatible adult living donor liver transplantation. Liver Transpl. 2007;13:579-588. [Cited in This Article: ] |
| 25. | Hori T, Uemoto S, Takada Y, Oike F, Ogura Y, Ogawa K, Miyagawa-Hayashino A, Yurugi K, Nguyen JH, Hori Y. Does a positive lymphocyte cross-match contraindicate living-donor liver transplantation? Surgery. 2010;147:840-844. [Cited in This Article: ] |
| 26. | Austin GL, Sasaki AW, Zaman A, Rabkin JM, Olyaei A, Ruimy R, Orloff SL, Ham J, Rosen HR. Comparative analysis of outcome following liver transplantation in US veterans. Am J Transplant. 2004;4:788-795. [Cited in This Article: ] |
| 27. | Yao FY, Ferrell L, Bass NM, Bacchetti P, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: comparison of the proposed UCSF criteria with the Milan criteria and the Pittsburgh modified TNM criteria. Liver Transpl. 2002;8:765-774. [Cited in This Article: ] |
| 28. | Park JB, Kwon CH, Choi GS, Chun JM, Jung GO, Kim SJ, Joh JW, Lee SK. Prolonged cold ischemic time is a risk factor for biliary strictures in duct-to-duct biliary reconstruction in living donor liver transplantation. Transplantation. 2008;86:1536-1542. [Cited in This Article: ] |
| 29. | Rana A, Hardy MA, Halazun KJ, Woodland DC, Ratner LE, Samstein B, Guarrera JV, Brown RS Jr, Emond JC. Survival outcomes following liver transplantation (SOFT) score: a novel method to predict patient survival following liver transplantation. Am J Transplant. 2008;8:2537-2546. [Cited in This Article: ] |
| 30. | Mor E, Jennings L, Gonwa TA, Holman MJ, Gibbs J, Solomon H, Goldstein RM, Husberg BS, Watemberg IA, Klintmalm GB. The impact of operative bleeding on outcome in transplantation of the liver. Surg Gynecol Obstet. 1993;176:219-227. [Cited in This Article: ] |
| 31. | Ben-Haim M, Emre S, Fishbein TM, Sheiner PA, Bodian CA, Kim-Schluger L, Schwartz ME, Miller CM. Critical graft size in adult-to-adult living donor liver transplantation: impact of the recipient’s disease. Liver Transpl. 2001;7:948-953. [Cited in This Article: ] |
| 32. | Yao FY, Saab S, Bass NM, Hirose R, Ly D, Terrault N, Lazar AA, Bacchetti P, Ascher NL, Roberts JP. Prediction of survival after liver retransplantation for late graft failure based on preoperative prognostic scores. Hepatology. 2004;39:230-238. [Cited in This Article: ] |
| 33. | Rhee C, Narsinh K, Venick RS, Molina RA, Nga V, Engelhardt R, Martín MG. Predictors of clinical outcome in children undergoing orthotopic liver transplantation for acute and chronic liver disease. Liver Transpl. 2006;12:1347-1356. [Cited in This Article: ] |
| 34. | Iida T, Yagi S, Taniguchi K, Hori T, Uemoto S, Yamakado K, Shiraishi T. Significance of CT attenuation value in liver grafts following right lobe living-donor liver transplantation. Am J Transplant. 2005;5:1076-1084. [Cited in This Article: ] |
| 35. | Rerolle JP, Akposso K, Lerolle N, Mougenot B, Ponnelle T, Rondeau E, Sraer JD. Tacrolimus-induced hemolytic uremic syndrome and end-stage renal failure after liver transplantation. Clin Transplant. 2000;14:262-265. [Cited in This Article: ] |
| 36. | Singh N, Gayowski T, Marino IR. Hemolytic uremic syndrome in solid-organ transplant recipients. Transpl Int. 1996;9:68-75. [Cited in This Article: ] |
| 37. | Ramasubbu K, Mullick T, Koo A, Hussein M, Henderson JM, Mullen KD, Avery RK. Thrombotic microangiopathy and cytomegalovirus in liver transplant recipients: a case-based review. Transpl Infect Dis. 2003;5:98-103. [Cited in This Article: ] |
| 38. | Akamatsu N, Sugawara Y, Tamura S, Togashi J, Kaneko J, Makuuchi M. Late mortality from thrombotic microangiopathy after liver transplantation: report of a case. Surg Today. 2007;37:345-348. [Cited in This Article: ] |
| 39. | Oya H, Sato Y, Yamamoto S, Nakatsuka H, Kobayashi T, Watanabe T, Kokai H, Hatakeyama K. Thrombotic microangiopathy after ABO-incompatible living donor liver transplantation: a case report. Transplant Proc. 2008;40:2549-2551. [Cited in This Article: ] |
| 40. | Takada Y, Haga H, Ito T, Nabeshima M, Ogawa K, Kasahara M, Oike F, Ueda M, Egawa H, Tanaka K. Clinical outcomes of living donor liver transplantation for hepatitis C virus (HCV)-positive patients. Transplantation. 2006;81:350-354. [Cited in This Article: ] |
| 41. | Saito T, Egawa H, Kudo T, Takakura S, Fujihara N, Iinuma Y, Ichiyama S. Pre-transplant risk factors predicting post-transplant cytomegalovirus infection in liver transplant recipients. Transpl Int. 2007;20:419-424. [Cited in This Article: ] |
| 42. | Baid S, Pascual M, Williams WW Jr, Tolkoff-Rubin N, Johnson SM, Collins B, Chung RT, Delmonico FL, Cosimi AB, Colvin RB. Renal thrombotic microangiopathy associated with anticardiolipin antibodies in hepatitis C-positive renal allograft recipients. J Am Soc Nephrol. 1999;10:146-153. [Cited in This Article: ] |
| 43. | Matsukura T, Yokoi A, Egawa H, Kudo T, Kawashima M, Hirata Y, Tanaka H, Kagajo K, Wada H, Tanaka K. Significance of serial real-time PCR monitoring of EBV genome load in living donor liver transplantation. Clin Transplant. 2002;16:107-112. [Cited in This Article: ] |
| 44. | Ueda M, Uemoto S, Inomata Y, Okajima H, Hashida T, Tanaka K, Yamaoka Y. A proposal of FK506 optimal dosing in living related liver transplantations. Transplantation. 1995;60:258-264. [Cited in This Article: ] |
| 45. | Bell WR, Braine HG, Ness PM, Kickler TS. Improved survival in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Clinical experience in 108 patients. N Engl J Med. 1991;325:398-403. [Cited in This Article: ] |
| 46. | Hori T, Egawa H, Takada Y, Oike F, Ogura Y, Ogawa K, Kaido T, Toshimitsu Y, Yagi S, Iida T. Fatal impact of lymphocyte cross-matching upon humoral rejection after adult living related liver transplantation. Transpl Int. 2010;23:338-340. [Cited in This Article: ] |
| 47. | Nakazawa Y, Hashikura Y, Urata K, Ikegami T, Terada M, Yagi H, Ishizashi H, Matsumoto M, Fujimura Y, Miyagawa S. Von Willebrand factor--cleaving protease activity in thrombotic microangiopathy after living donor liver transplantation: a case report. Liver Transpl. 2003;9:1328-1333. [Cited in This Article: ] |
| 48. | Banno S, Ito Y, Tanaka C, Hori T, Fujimoto K, Suzuki T, Hashimoto T, Ueda R, Mizokami M. Quantification of red blood cell fragmentation by the automated hematology analyzer XE-2100 in patients with living donor liver transplantation. Clin Lab Haematol. 2005;27:292-296. [Cited in This Article: ] |