Published online Feb 14, 2010. doi: 10.3748/wjg.v16.i6.703
Revised: November 12, 2009
Accepted: November 19, 2009
Published online: February 14, 2010
AIM: To investigate the therapeutic effect of Schistosoma mansoni (S. mansoni) soluble worm proteins on gastrointestinal motility disturbances during experimental colitis in mice.
METHODS: Colitis was induced by intrarectal injection of trinitrobenzene sulphate (TNBS) and 6 h later, mice were treated ip with S. mansoni proteins. Experiments were performed 5 d after TNBS injection. Inflammation was quantified using validated inflammation parameters. Gastric emptying and geometric center were measured to assess in vivo gastrointestinal motility. Peristaltic activity of distal colonic segments was studied in vitro using a modified Trendelenburg set-up. Cytokine profiles of T-lymphocytes isolated from the colon were determined by real time reverse transcriptase-polymerase chain reaction.
RESULTS: Intracolonic injection of TNBS caused severe colitis. Treatment with S. mansoni proteins significantly ameliorated colonic inflammation after 5 d. TNBS did not affect gastric emptying but significantly decreased the geometric center and impaired colonic peristaltic activity 5 d after the induction of colitis. Treatment with S. mansoni proteins ameliorated these in vivo and in vitro motility disturbances. In addition, TNBS injection caused a downregulation of effector T cell cytokines after 5 d, whereas a S. mansoni protein effect was no longer observed at this time point.
CONCLUSION: Treatment with S. mansoni proteins attenuated intestinal inflammation and ameliorated motility disturbances during murine experimental colitis.
-
Citation: Ruyssers NE, De Winter BY, De Man JG, Ruyssers ND, Van Gils AJ, Loukas A, Pearson MS, Weinstock JV, Pelckmans PA, Moreels TG.
Schistosoma mansoni proteins attenuate gastrointestinal motility disturbances during experimental colitis in mice. World J Gastroenterol 2010; 16(6): 703-712 - URL: https://www.wjgnet.com/1007-9327/full/v16/i6/703.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i6.703
Crohn’s disease and ulcerative colitis, the two most common forms of inflammatory bowel diseases (IBD), are idiopathic inflammatory disorders of the intestine. The current hypothesis states that IBD results from an uncontrolled immune response against intraluminal bacterial antigens in genetically predisposed individuals[1,2]. Genetic factors as well as environmental factors contribute to the development of the inappropriate immune response[3].
The incidence of Crohn’s disease is highest in well-developed countries[4]. According to the hygiene hypothesis, this is directly related to the higher hygienic standards in these countries[5]. It is suggested that the lack of exposure to intestinal parasites (e.g. helminths) contributes to the susceptibility to Crohn’s disease[6-8]. Several experimental and clinical studies showed the beneficial effect of helminth infections in IBD[9-12]. Current research is focusing on identifying helminth molecules with immunomodulatory function that exert this protective effect[13].
Patients with Crohn’s disease often suffer from disturbed gastrointestinal motility leading to symptoms such as abdominal pain, cramps, diarrhea, weight loss, rectal bleeding and malnutrition[14,15]. It is well established that inflammation of the gut results in functional and structural changes of the enteric nervous system[16,17] and changes in smooth muscle contractility[18,19]. For instance, patients with ulcerative colitis have increased propagating contractions with lower peak amplitudes coupled with variable transit[20], whereas delayed gastric emptying and prolongation of orocecal transit time have been reported in patients with Crohn’s disease[21-23]. Dysmotility can also occur in non-inflamed sites of the gastrointestinal tract. Gastroparesis often occurs in patients with inflammation restricted to the small or large intestine[14,24]. Motility disturbances may also persist in the period following an episode of gastrointestinal inflammation, resulting in the development of irritable bowel syndrome or functional dyspepsia[14,25].
The aim of this study was to investigate the therapeutic potential of Schistosoma mansoni (S. mansoni) soluble worm proteins (SmSWP) on gastrointestinal motility disturbances 5 d after induction of experimental colitis in mice both in vivo and in vitro. In addition, the inflammatory reaction was quantified and the balance between different T cell subsets was investigated based on their cytokine profile to elucidate the underlying immunological pathways.
Colitis was induced by intraluminal injection of 2,4,6-trinitrobenzene sulfonic acid (TNBS) as previously described[26]. Briefly, male Swiss mice (weight 26-28 g, Charles River, France) were fasted for 24 h and subsequently anesthetized by ketamine (90 mg/kg, ip) and xylazine (10 mg/kg, ip). Next, 100 μL of a 10 mg TNBS in 30% ethanol solution was injected intrarectally. Ethanol is required to break the intestinal epithelial barrier, whereas TNBS is a haptenating agent that immunogenizes autologous proteins. Control animals received an intrarectal injection of 100 μL saline. Afterwards, mice were held upside-down for 1 min to prevent leakage of TNBS solution. The Medical Ethical Committee on animal experimentation of the University of Antwerp, Belgium, approved all experiments.
SmSWP were prepared as described previously[27]. Briefly, S. mansoni adult worms were recovered from mice (housed at the Queensland Institute of Medical Research, Brisbane, Australia), washed and homogenized in PBS and soluble proteins were extracted by centrifugation. Mice were treated with 25 μg SmSWP ip. Proteins were diluted to a final volume of 100 μL in PBS. Control animals were injected ip with 100 μL PBS.
In a previous study we investigated the time course of inflammation during TNBS colitis and found that inflammation peaked at day 3 and that colitis was self-limiting with near complete remission after 1 wk. In the present study, we wanted to evaluate the effect of helminth protein treatment after the peak of inflammation but when overt signs of colitis were still present.
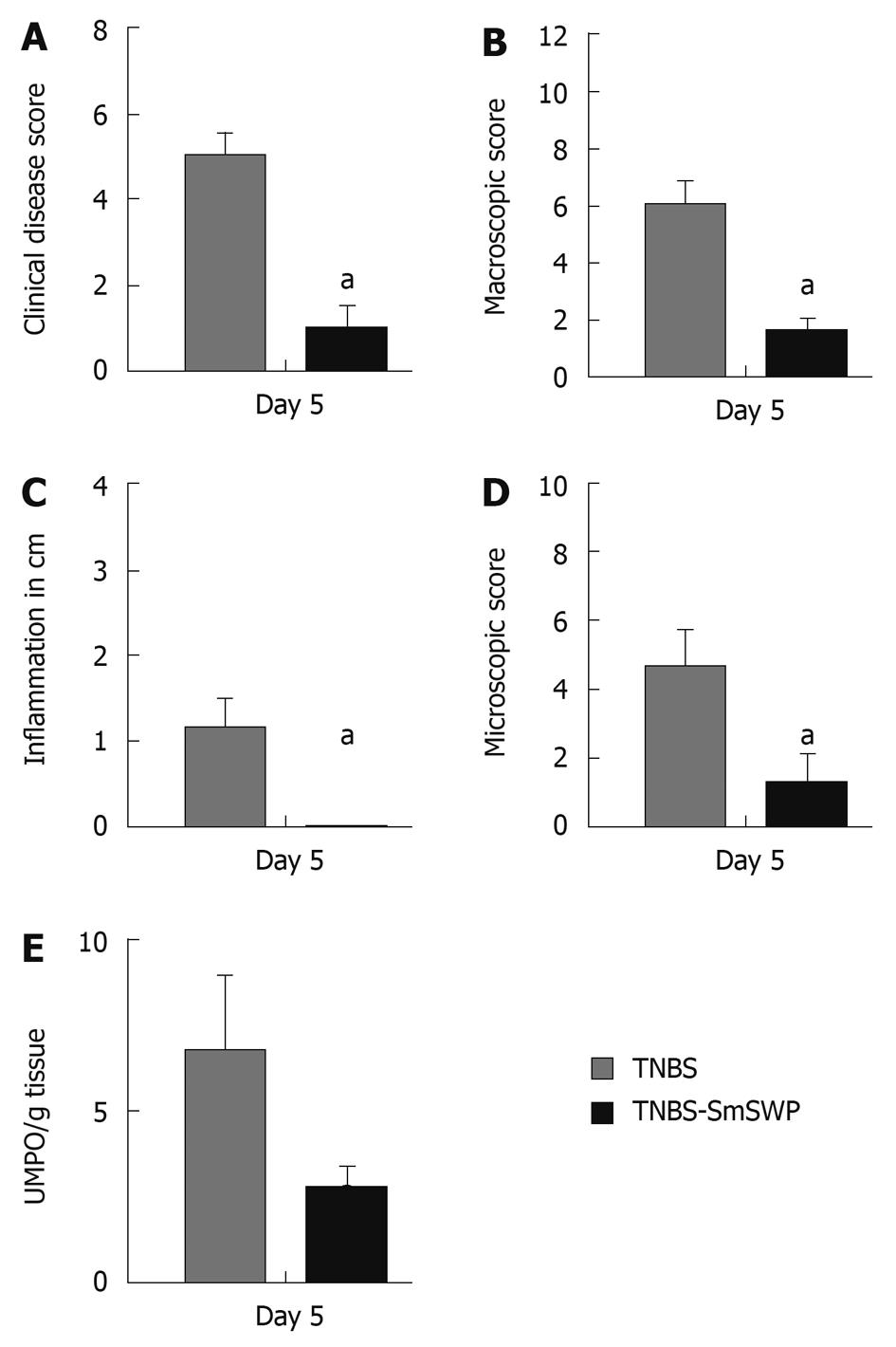
In a first set of experiments, we scored the therapeutic effect of SmSWP on colonic inflammation 5 d after the induction of colitis. Six hours after TNBS injection, mice were treated once ip with 25 μg S. mansoni proteins or phosphate-buffered saline (PBS). Five days later, mice were sacrificed and inflammation was scored based on 5 parameters: clinical disease activity, macroscopic and microscopic inflammation score, extent of colonic inflammation and myeloperoxidase activity. Two different groups were studied: TNBS mice treated with PBS (TNBS-PBS) after 5 d and TNBS mice treated with 25 μg SmSWP (TNBS-SmSWP) after 5 d (n = 8-10 in each group).
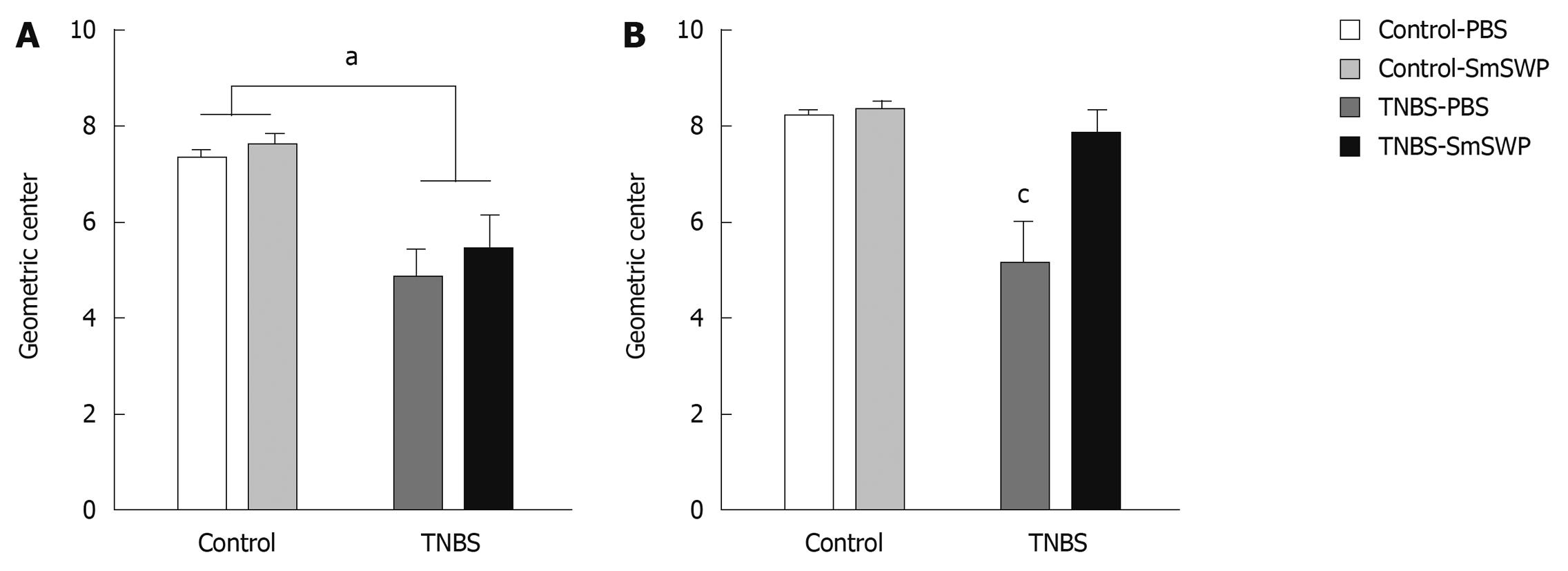
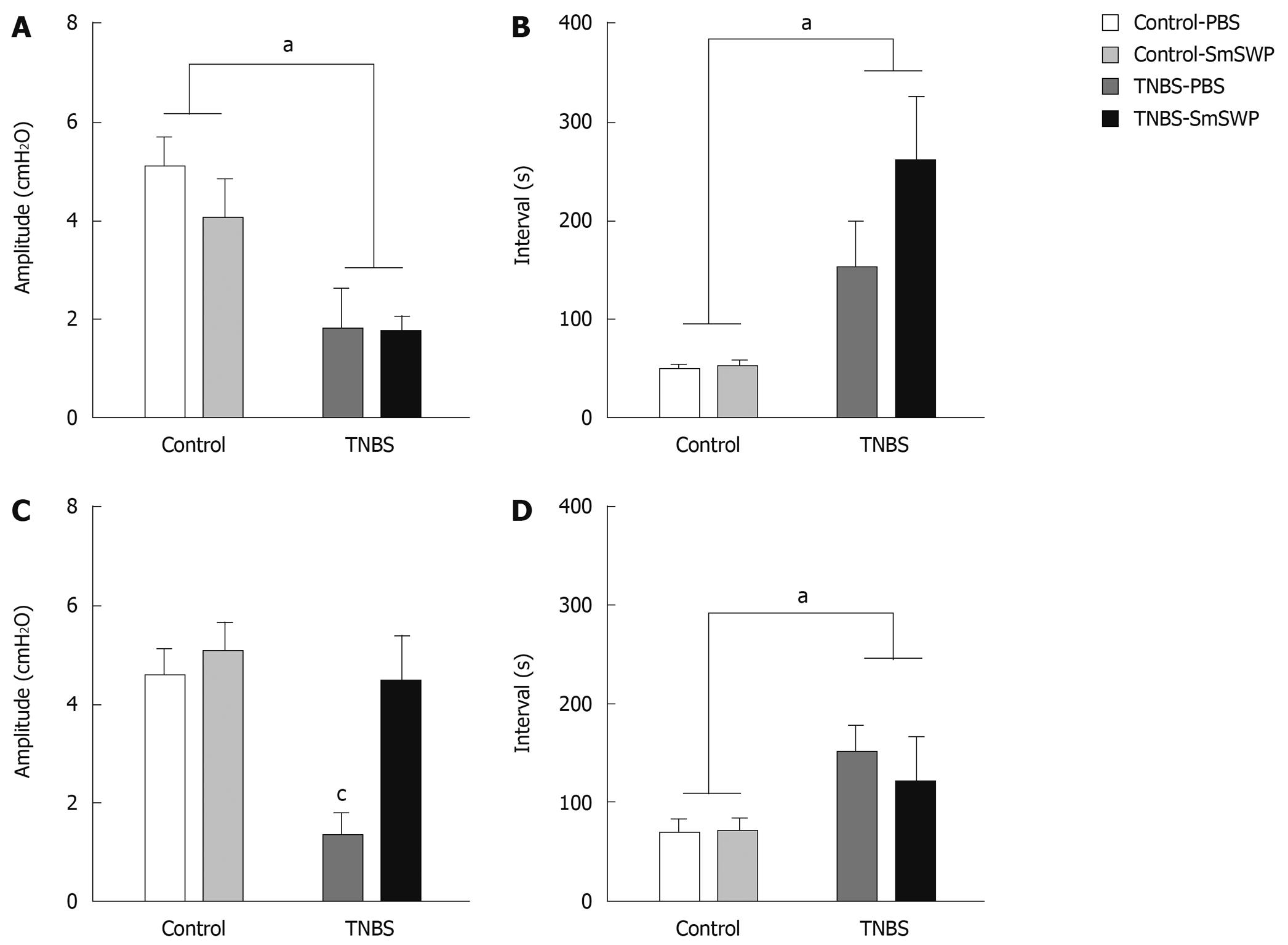
In a second set of experiments, we investigated the effect of SmSWP treatment on in vivo gastrointestinal motility and in vitro colonic peristalsis 5 d after induction of colitis. Four different groups were studied: control-PBS mice, control mice treated with 25 μg SmSWP (control-SmSWP), TNBS-PBS mice and TNBS-SmSWP mice (n = 7-10 in each group). In preliminary experiments we also investigated the effect of colitis on gastrointestinal motility disturbances 3 d after the induction of colitis, and the effect of SmSWP treatment as these experiments were not performed previously.
In a third set of experiments, we investigated the cytokine profile of colonic T cells 5 d after induction of colitis. Cytokine profiles were studied in 4 different groups: control-PBS, control-SmSWP, TNBS-PBS, TNBS-SmSWP (n = 5-8 in each group, for each n, colonic tissue of 3 mice was pooled).
Briefly, the clinical disease score (0-8) was based on the following characteristic parameters (0-2 score each): weight loss, piloerection, immobility and blepharitis[27].
After sacrifice, the colon was removed and opened to score colonic damage macroscopically. Four parameters were taken into account: presence of adhesions, degree of colonic ulcerations, wall thickness, and degree of mucosal edema. The total score ranged from 0 to 12[28]. The extent of inflammation in the colon was also measured and expressed in cm. Tissue samples were harvested for histological assessment of the inflammatory infiltrate and for myeloperoxidase (MPO) assay. Colonic segments were fixed in 4% formaldehyde and embedded in paraffin for hematoxylin-eosin staining. Microscopic inflammation score ranged from 0 to 10 based on the following parameters: inflammatory infiltrate, number of gut wall layers infiltrated, loss of mucosal architecture, and edema[27]. MPO activity was measured to monitor the degree of myeloid cell infiltration in the colon. Colonic MPO activity was assayed according to published methods[29] and expressed as units MPO per gram tissue.
Mice were fasted for 18 h and in vivo semi-liquid meal motility was assessed according to published methods[30]. Briefly, mice received an intragastric injection of 0.1 mL Evans blue (50 mg/mL + 0.5% methylcellulose) via an orogastric cannula. Fifteen minutes later, mice were anesthetized and a laparotomy was performed. The stomach and small intestine were resected and the small intestine was divided into 5 segments of equal length. The amount of Evans blue in the segments was measured spectrophotometrically to assess gastric emptying (GE) and geometric center (GC): %GE = [ΣA565 (intestine 1-5)/ΣA565 (stomach + intestine 1-5)] × 100; GC = Σ (A565 of Evans blue per segment × segment number)/total A565.
Mice were fasted for 18 h and in vivo solid meal motility was assessed as previously described[31]. Mice received an intragastric gavage of 25 green glass beads (0.4-0.5 mm in diameter) together with 0.5 mL H2O solution via an orogastric cannula and were transferred to a wired bottom cage to prevent coprophagy[32]. Subsequently, 30, 120 and 360 min after gavage, mice were anesthetized, the gastrointestinal tract was resected and divided into different segments: stomach, 5 small intestinal segments, cecum, 2 colonic segments and feces. The number of beads in each segment was counted under a stereomicroscope and GE and GC were calculated by the following equations: %GE = [number of beads (small intestine 1-5 + cecum + colon 1-2 + feces)/total number of beads] × 100; GC = Σ (beads per segment × segment number)/total number of beads.
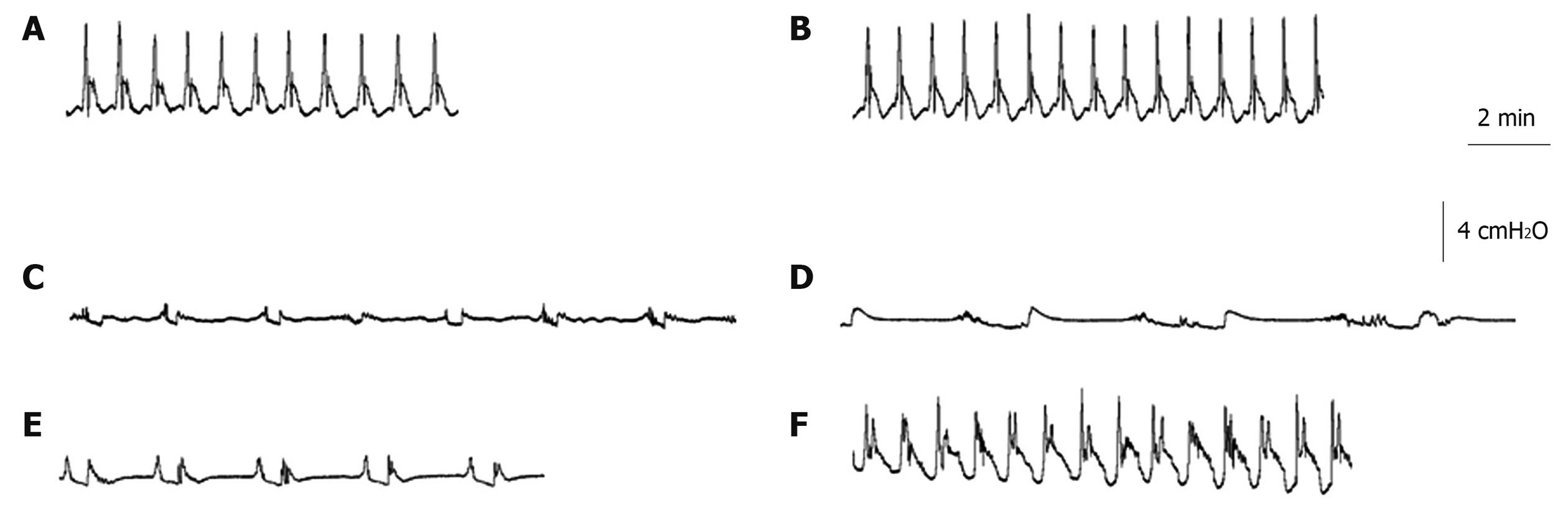
Assessment of colonic peristalsis was performed as previously described[31]. Briefly, mice were anesthetized, the colon was removed, flushed and put in cold aerated Krebs-ringer solution. The distal colon segment (3 cm in length) was mounted horizontally in an organ bath. For each segment, the oral end was connected to a perfusion pump for intraluminal infusion of Krebs solution and the other end was attached to a pressure transducer and a vertical tube of which the outlet could be raised in height. After 30 min of equilibration, the outlet was increased from 0 to 7.5 cm. Under these circumstances, spontaneous peristaltic contractions occurred. This activity was associated with regular pressure increases which were recorded by the pressure transducer and analyzed by a data-acquisition system (CED 1401, Cambridge Electronic Design, Cambridge, UK). After an equilibration period of 20 min, the mean amplitude (cmH2O) of 3 consecutive peristaltic contractions as well as the mean time interval(s) between 4 subsequent peristaltic contractions were calculated and compared.
Colonic lamina propria mononuclear cells were isolated based on a 30%:70% gradient Percoll column as previously described[27,33]. Colonic lamina propria T cells were subsequently isolated by positive selection using the EasySep enrichment procedure employing antibody-coated, magnetic particles as described by the manufacturer (Stem Cell Technologies, Vancouver, Canada). Total RNA was extracted from isolated colonic T cells by using the Absolutely RNA microprep kit as described by the manufacturer (Stratagene, La Jolla, CA, USA).
Using real time reverse transcriptase-polymerase chain reaction (RT-PCR), we performed a quantitative analysis of the mRNA expression of different cytokines to determine the balance between T helper (Th) 1, Th17, Th2 and Treg (regulatory T) cells in colonic tissue. TaqMan Gene Expression assays (Applied Biosystems, Lennik, Belgium) specific for IFNγ produced by Th1 cells, IL17 produced by Th17 cells, IL-5 produced by Th2 cells and IL-10 produced by Treg cells were performed on a ABI Prism 7300 sequence detector system (Applied Biosystems, Lennik, Belgium) in 25 μL reaction volumes containing One step Universal PCR master mix (Applied Biosystems, Lennik, Belgium) as previously described[27].
NaCl 0.9% (Plurule®, Baxter, Lessines, Belgium); 2,4,6 trinitrobenzene sulfonic acid solution (Fluka, Neu-Ulm, Germany); PBS (GIBCO BRL, Merelbeke, Belgium); diethyl ether, ethanol absolute, 30% hydrogen peroxide, methanol absolute, potassium dihydrogen phosphate, dipotassium hydrogen phosphate trihydrate (Merck, Darmstadt, Germany); xylazine (Rompun®, Bayer, Brussels, Belgium); ketamine (Ketalar®, Pfizer, Brussels, Belgium); hexadecyltrimethylammonium bromide, o-dianisidine dihydrochloride, FCS, collagenase, Percoll, Evans blue (Sigma Chemical, St. Louis, Missouri, USA); RPMI 1640, EDTA, HBSS, HEPES, L-glutamine, β-mercaptoethanol, sodium pyruvate, penicillin, streptomycin (Invitrogen, Merelbeke, Belgium), glass beads (0.4-0.5 mm in diameter) (VWR international, Leuven, Belgium) were purchased from the respective companies mentioned in parentheses. Helminth protein preparation was described earlier.
Data are presented as mean ± SE. Statistical analysis was performed in SPSS 16.0 for Windows. Analyses of the non-parametric data (clinical disease score, macroscopic and microscopic inflammation score) were performed by Mann-Whitney U tests. Parametric data (extent of inflammation, MPO, GE, GC, amplitude, interval and RT-PCR results) were analyzed by Student’s t-tests or by two-way ANOVA (with TNBS colitis as factor 1 and protein treatment as factor 2). When the interaction was significant one-way ANOVA and Student-Newman-Keuls post hoc analysis was performed. P values ≤ 0.05 were considered to be significant.
The injection of TNBS caused an increase in all inflammatory parameters (Figure 1A-E) as compared to control mice that did not show any signs of inflammation (data not shown).
Treatment of TNBS-injected mice with SmSWP caused a significant decrease in clinical disease score (Figure 1A), macroscopic inflammation score (Figure 1B), extent of colonic inflammation (Figure 1C), microscopic inflammation score (Figure 1D) and a tendency to decrease the MPO activity (Figure 1E) as compared to TNBS-PBS mice.
In preliminary experiments, 3 d after the induction of colitis, GE and GC of a semi-liquid Evans blue solution were not significantly different between control and TNBS mice: GE was 43% ± 9% in controls and 48% ± 10% in TNBS colitis mice and GC was 2.1 ± 0.3 in controls and 2.2 ± 0.3 in TNBS colitis mice (n = 7-9). We also evaluated the effect of colitis on gastrointestinal motility after intragastric gavage of 25 glass beads 3 d after the injection of TNBS. Experiments were performed 30 min, 120 min and 360 min after intragastric gavage of the marker: GE progressed over time in control mice (from 32% ± 12% to 61% ± 14% and 100% ± 0%, respectively) and in TNBS mice (from 42% ± 13% to 81% ± 9% and 97% ± 2%, respectively) but no significant differences between the control and TNBS groups were observed. The GC also increased over time from 1.5 ± 0.2 (30 min) to 3.2 ± 0.7 (120 min) and to 7.3 ± 0.2 (360 min) in control mice. This time-dependent increase in GC was also observed in mice with colitis (from 1.7 ± 0.3 to 2.9 ± 0.5 and 5.5 ± 0.6). When measured 360 min after gavage of the beads, GC in mice with colitis (5.5 ± 0.6) was significantly lower as compared to control mice (7.33 ± 0.2). Based on these preliminary results, further measurements studying the effect of worm protein treatment on GC were performed 360 min after intragastric gavage of 25 glass beads.
Experiments were performed 3 d (Figure 2A) and 5 d (Figure 2B) after TNBS injection. Treatment of control mice with SmSWP had no effect per se on GC at both time points (Figure 2A and B). TNBS-colitis significantly reduced GC 360 min after intragastric gavage of the beads both at day 3 and at day 5 (Figure 2A and B). Treatment of colitis mice with SmSWP had no effect on GC 3 d after the injection of TNBS (Figure 2A). However, treatment of colitis mice with SmSWP reversed the TNBS-induced decrease in GC at day 5 (Figure 2B).
Distention-induced peristaltic contractions were recorded (Figure 3) and, subsequently, the amplitude and the interval between the peristaltic waves were measured.
Peristaltic activity of distal colonic segments was measured 3 d (Figure 4A and B) and 5 d (Figure 4C and D) after TNBS enema. Treatment of control mice with SmSWP had no significant effect on colonic peristaltic activity at the two different time points (Figure 3B and Figure 4A-D). The induction of colitis caused significant impairment of peristaltic activity as shown by a significant decrease in amplitude and an increase in interval between the waves. These TNBS-induced disturbances in peristalsis were significant both on day 3 and on day 5 (Figure 3C and D, Figure 4A-D). Furthermore, it is important to note that in 4 of 8 TNBS-PBS mice on day 3 we were not able to measure any peristaltic activity whereas this was only the case in 1 of 8 TNBS-SmSWP mice.
Treatment with SmSWP did not ameliorate the disturbed peristaltic activity caused by intestinal inflammation after 3 d (Figure 3E, Figure 4A and B). However, 5 d after the induction of colitis the amplitude of the distension-induced peristaltic contractions was significantly increased to normal control values when mice were treated with SmSWP (Figure 3F and Figure 4C). At this time point, the mean interval between the waves remained increased after treatment with SmSWP as compared to control animals (Figure 4D).
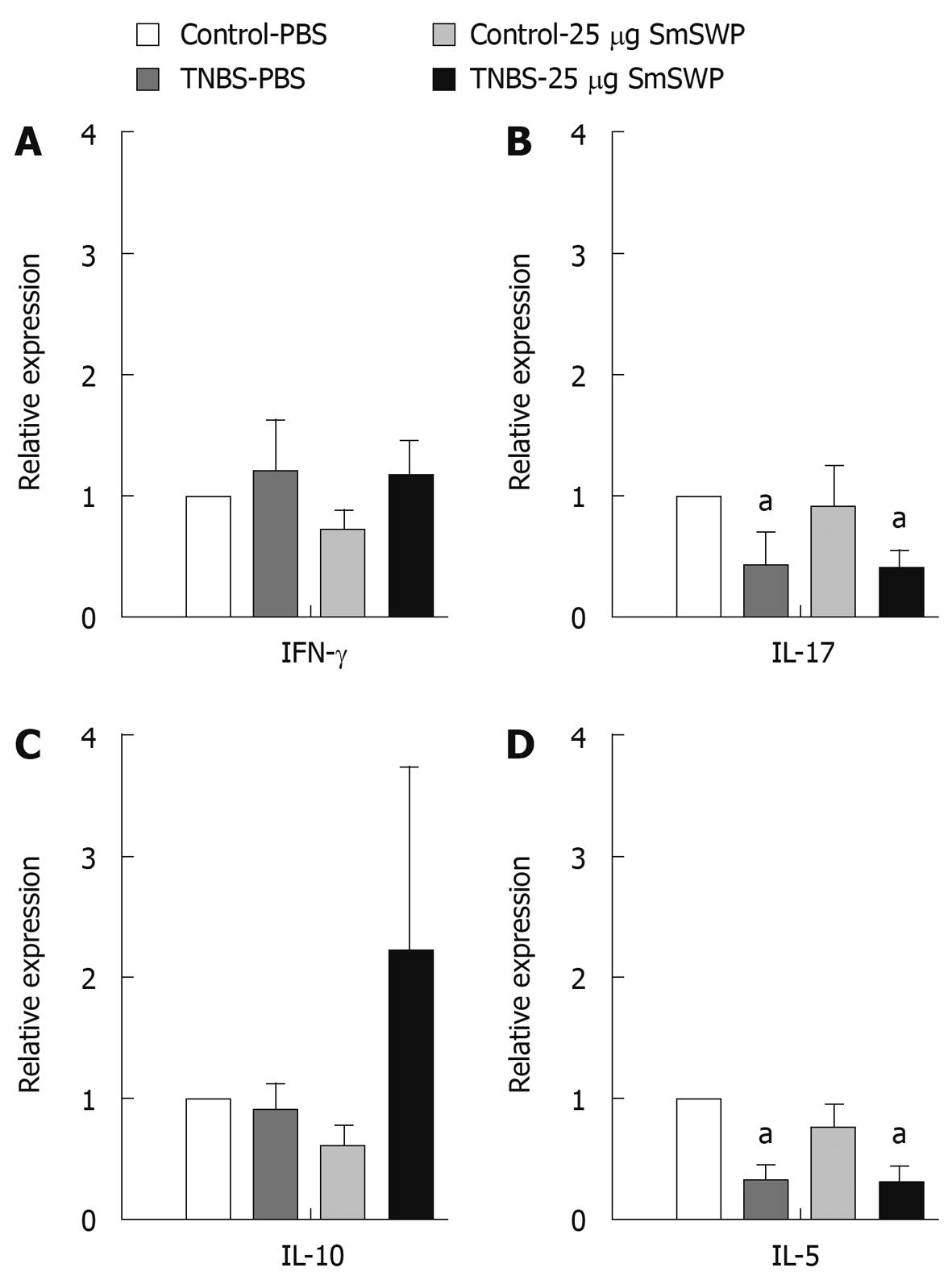
We recently showed the importance of the differential roles of Th1, Th17, Th2 and Treg cells in colonic tissue 3 d after the induction of TNBS colitis and the effect of SmSWP treatment on these T cell subsets[27]. In this study we investigated the cytokine profiles of T cells isolated from colonic tissue on day 5 after the induction of colitis. As shown in Figure 5A, interferon (IFN)-γ mRNA expression was not significantly altered in colonic T cells 5 d after the induction of colitis. On the other hand, we found a significant downregulation of interleukin (IL)-17 and IL-5 expression 5 d after the induction of colitis in both PBS- and SmSWP-treated mice (Figure 5B and D). Investigating the Treg response, we found that injection of TNBS and treatment with SmSWP had no significant effect on IL-10 mRNA expression 5 d after TNBS injection (Figure 5C).
In this study we showed that treatment with SmSWP ameliorated in vivo and in vitro motility disturbances in a murine model of TNBS-induced colitis after 5 d.
Experimental and clinical data support the idea that helminths provide protection against IBD[34]. To avoid the possible disadvantages of a therapy with living parasites, current research is now focusing on the identification and characterization of helminth-derived immunosuppressive molecules that contribute to the protective effect[13]. Furthermore, it is well established that gut inflammation leads to disturbed gastrointestinal motility[14]. The model of TNBS-induced colitis is widely used to investigate motility disturbances occurring in the inflamed colon. We previously showed that contractility of colonic longitudinal smooth muscle strips was time-dependently decreased during TNBS colitis in rats and concurrent infection with S. mansoni abrogated these TNBS-induced contractility disturbances[10].
In a previous study we showed that TNBS colitis caused clear signs of inflammation 3 d after the induction of colitis and that our model of TNBS colitis was self-limiting with near complete remission after 1 wk[27]. In this study we focused on a later phase during murine TNBS-induced colitis. We showed that 5 d after TNBS injection, treatment with SmSWP also caused a significant decrease in clinical disease score, macroscopic inflammation score, extent of colonic inflammation and microscopic inflammation score at this later time point along the course of colitis. There was however no significant difference in MPO activity between TNBS-PBS and TNBS-SmSWP mice at this time point although a clear tendency of inhibition was observed. Taken together, these results indicate that treatment with helminth proteins ameliorated colonic inflammation leading to accelerated healing of colitis. A similar beneficial effect has been described previously by our group: rats with TNBS colitis showed spontaneous and complete healing of inflammation 4 wk after the induction of colitis and this was reduced to 2 wk in rats infected with S. mansoni[10].
Investigation of the effect of TNBS colitis on gastrointestinal motility failed to show an effect on gastrointestinal transit of a semi-liquid meal. In other words, colitis did not affect gastric emptying in our murine model. Nevertheless, delayed gastric emptying has been described in a clinical setting[21,23] as well as in the rat TNBS model[35]. Literature on TNBS-induced motility disturbances in mice is scarce and gastric emptying disturbances have not been reported so far. In addition to species differences, this lack of effect of colitis on gastric emptying in mice might be linked to the type and severity of inflammation induced and to the time point chosen to perform motility experiments.
On the other hand, assessment of gastrointestinal transit of a solid meal showed that the geometric distribution of solid beads in the gastrointestinal tract was significantly decreased 3 d after the induction of colitis and this decrease was still evident after helminth protein treatment by day 3. Five days after the induction of colitis, the geometric distribution was still significantly altered in mice with colitis but treatment with worm proteins significantly reversed transit to normal values at this time point. These results indicate that although the healing process of intestinal inflammation is ongoing in untreated TNBS mice by day 5, gastrointestinal motility of the gastrointestinal tract remains disturbed. Only when inflammatory signs are almost completely absent, as in the SmSWP-treated TNBS mice on day 5, was in vivo gastrointestinal motility of the distal gastrointestinal tract restored.
Comparable results were found on in vitro colonic peristalsis. The amplitude of distension-induced pressure waves as well as the interval between the waves were significantly altered in the colon of mice with TNBS-induced colitis, both at day 3 and day 5. Treatment with SmSWP did not have any ameliorating effect after 3 d whereas the amplitude was significantly increased to normal control values after 5 d. The interval between the waves was nevertheless still significantly augmented as compared to controls at day 5. This suggests that some signs of disturbed peristalsis persisted although inflammation is resolving and that the disturbed interval of in vitro peristaltic waves 5 d after colitis and worm treatment did not have any repercussion on in vivo colonic motility which, at that time point, was normalized in these mice.
With regard to the clinical setting, treatment of IBD patients with Trichuris suis ova caused clinical amelioration of both Crohn’s disease and ulcerative colitis as shown by a decrease in Crohn’s disease activity index and ulcerative colitis disease activity index[11,12]. This decrease in clinical disease scores might indicate that symptoms such as diarrhea and abdominal pain are less frequent after treatment with helminths. It is well known that infection with intestinal helminths can alter gastrointestinal motility thus contributing to worm expulsion[36]. The role of T cells in those circumstances was previously investigated, leading to the understanding that infection-induced intestinal muscle hypercontractility is CD4+ T cell-dependent[37].
Cytokines produced by mucosal leucocytes can also mediate neurogastrointestinal function. We previously showed that the pro-inflammatory cytokine IL-1β modulates gastrointestinal neuromuscular function[38]. In addition, Th2 cytokines IL-4 and IL-13 contribute to intestinal muscle hypercontractility[39]. Treatment with exogenous IL-10 has been shown to abrogate the delayed gastrointestinal transit during postoperative ileus[40]. Gastrointestinal inflammation during Crohn’s disease is mediated via Th1 lymphocytes as well as through the recently described Th17 cells[41]. On the other hand, it is well established that helminths have the potential to evoke strong regulatory T cell responses with immunosuppressive properties[42]. In this way, we might hypothesize that infection with helminths induce Th2 and Treg immune responses that contribute to the amelioration of motility disturbances during colitis.
As such, we measured the cytokine profile of colonic T cells. We previously showed that a Th1 response (upregulation of IFN-γ) in the colon was evident 3 d after induction of TNBS colitis. This Th1 response was significantly suppressed after administration of S. mansoni proteins. Treatment with SmSWP also caused an upregulation of regulatory T cell cytokines in the colon after 3 d[27]. In this study we identified the balance between the different T cell subsets in the colon at a later time point along the course of colitis, 5 d after the injection of TNBS. Our results showed there was no longer a significant effect on IFN-γ mRNA expression after the induction of colitis, indicating that the Th1 response seen on day 3 in the colon of TNBS-PBS mice had subsided by day 5. Furthermore, injection of helminth proteins decreased the expression of IL-17 after 3 d, both in control mice and in TNBS mice[27]. After 5 d we found decreased IL-17 mRNA expression due to a colitis effect instead of a protein effect. These differential results on IL-17 mRNA expression at both time points are interesting: at day 3 the effect on IL-17 expression was related to helminth protein, whereas it was colitis mediated at day 5. Although we did not detect a significant effect of colitis or worm protein treatment on IL-5 expression after 3 d, a significant downregulation of IL-5 expression on day 5 was apparent both in the PBS treated group and in the helminth protein treated group. One might hypothesize that the naturally occurring healing response leads to the production of regulatory cytokines which are able to suppress cytokines produced by T effector cells including IL-17 and IL-5. This coincides with the attenuation of inflammatory parameters at this time point as described above.
Experiments performed 3 d after the induction of colitis showed a significant upregulation of the mRNA expression of regulatory cytokines IL-10 and transforming growth factor-β after treatment with SmSWP that had subsided by day 5. Our results showed that the immunological effect of helminth protein treatment on Th1 and Treg cells, which is present after 3 d as shown previously[27], has diminished after 5 d. This might be explained by the fact that proteins were only injected once 6 h after TNBS or PBS injection and not repeatedly until day 5. Nevertheless, this single injection with helminth proteins evoked a protective effect that was almost immediate, leading us to assume that these proteins might also have an effect on innate immunity. It was previously reported that infection with S. mansoni prevented experimental colitis in mice by a mechanism dependent on macrophages[43]. Furthermore, dendritic cells are key regulators in the immune defence of the gut and are also influenced by helminth infections[44,45]. Investigation on how helminth proteins affect cells of the innate immune system might contribute to a better understanding of the immunological pathways by which helminth proteins suppress ongoing colonic inflammation.
In this study, we provide evidence that treatment with helminth proteins contributes to amelioration of gastrointestinal motility disturbances. Inhibition of inflammation and amelioration of motility disturbances after treatment with helminth proteins both appear at the same time. However, whether the beneficial effect of helminth protein treatment on gastrointestinal motility is directly or indirectly related to amelioration of inflammation needs to be further established. If helminth proteins provoke a reaction that not only leads to a reduction in inflammation but also influences the enteric nervous system and/or smooth muscle cells directly, these proteins might be useful in the treatment of gastrointestinal motility disturbances.
Taken together, we showed that treatment with S. mansoni proteins significantly attenuated the course of TNBS-induced colitis leading to reversal of in vivo gastrointestinal motility disturbances and amelioration of in vitro colonic peristalsis 5 d after induction. We conclude that SmSWP have therapeutic potential in gut inflammation leading to a marked reduction in inflammation and in gastrointestinal motility disturbances, accelerating the natural course of remission.
Gastrointestinal inflammation during inflammatory bowel diseases (IBD) results from an uncontrolled immune response against intraluminal antigens in genetically predisposed persons and might lead to motility disturbances with related symptoms. The lack of exposure to helminth infections, as a result of improved living standards and medical conditions, has contributed to the increased incidence of IBD in the developed world.
Epidemiological, experimental and clinical data support the idea that helminths provide protection against IBD. However, treatment with living helminths may have serious drawbacks such as infection and/or invasion of the parasite to other tissues in the human host where they might cause pathology. Therefore, in this study the authors evaluated the therapeutic potential of helminth-derived proteins on inflammation and associated motility disturbances.
This study investigates the effect of TNBS colitis and exposure to Schistosoma mansoni proteins on murine gastrointestinal motility. This is a novel pursuit. The effects of inflammation and therapeutic interventions on gastrointestinal motility are largely ignored but critically important. The authors showed that treatment of experimental colitis with helminth proteins restored gastrointestinal motility.
Treatment with helminth soluble proteins attenuates inflammation and ameliorates motility disturbances during experimental colitis. These results suggest that helminth soluble proteins represent an attractive therapeutic option in the management of IBD.
This is an interesting study dealing with the effect of Schistosoma mansoni proteins on inflammatory and motility response in a rat model of inflammatory bowel disease. The experimental methods are described comprehensively and the interpretations and conclusions justified by the results.
Peer reviewer: Angelo A Izzo, Professor, Department of Experimental Pharmacology, University of Naples Federico II, via D Montesano 49, Naples 80131, Italy
S- Editor Wang YR L- Editor Cant MR E- Editor Ma WH
| 1. | Bamias G, Cominelli F. Novel strategies to attenuate immune activation in Crohn’s disease. Curr Opin Pharmacol. 2006;6:401-407. [Cited in This Article: ] |
| 2. | Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417-429. [Cited in This Article: ] |
| 3. | Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182-205. [Cited in This Article: ] |
| 4. | Alic M. Inflammatory bowel diseases are diseases of higher socioeconomic status: dogma or reality? Am J Gastroenterol. 2000;95:3332-3333. [Cited in This Article: ] |
| 5. | Strachan DP. Family size, infection and atopy: the first decade of the “hygiene hypothesis”. Thorax. 2000;55 Suppl 1:S2-S10. [Cited in This Article: ] |
| 6. | Lashner BA, Loftus EV Jr. True or false? The hygiene hypothesis for Crohn’s disease. Am J Gastroenterol. 2006;101:1003-1004. [Cited in This Article: ] |
| 7. | Weinstock JV, Summers RW, Elliott DE, Qadir K, Urban JF Jr, Thompson R. The possible link between de-worming and the emergence of immunological disease. J Lab Clin Med. 2002;139:334-338. [Cited in This Article: ] |
| 8. | Moreels TG, Pelckmans PA. Gastrointestinal parasites: potential therapy for refractory inflammatory bowel diseases. Inflamm Bowel Dis. 2005;11:178-184. [Cited in This Article: ] |
| 9. | Elliott DE, Li J, Blum A, Metwali A, Qadir K, Urban JF Jr, Weinstock JV. Exposure to schistosome eggs protects mice from TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2003;284:G385-G391. [Cited in This Article: ] |
| 10. | Moreels TG, Nieuwendijk RJ, De Man JG, De Winter BY, Herman AG, Van Marck EA, Pelckmans PA. Concurrent infection with Schistosoma mansoni attenuates inflammation induced changes in colonic morphology, cytokine levels, and smooth muscle contractility of trinitrobenzene sulphonic acid induced colitis in rats. Gut. 2004;53:99-107. [Cited in This Article: ] |
| 11. | Summers RW, Elliott DE, Urban JF Jr, Thompson R, Weinstock JV. Trichuris suis therapy in Crohn’s disease. Gut. 2005;54:87-90. [Cited in This Article: ] |
| 12. | Summers RW, Elliott DE, Urban JF Jr, Thompson RA, Weinstock JV. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology. 2005;128:825-832. [Cited in This Article: ] |
| 13. | Ruyssers NE, De Winter BY, De Man JG, Loukas A, Herman AG, Pelckmans PA, Moreels TG. Worms and the treatment of inflammatory bowel disease: are molecules the answer? Clin Dev Immunol. 2008;2008:567314. [Cited in This Article: ] |
| 14. | De Schepper HU, De Man JG, Moreels TG, Pelckmans PA, De Winter BY. Review article: gastrointestinal sensory and motor disturbances in inflammatory bowel disease - clinical relevance and pathophysiological mechanisms. Aliment Pharmacol Ther. 2008;27:621-637. [Cited in This Article: ] |
| 15. | Kraneveld AD, Rijnierse A, Nijkamp FP, Garssen J. Neuro-immune interactions in inflammatory bowel disease and irritable bowel syndrome: future therapeutic targets. Eur J Pharmacol. 2008;585:361-374. [Cited in This Article: ] |
| 16. | Collins SM. The immunomodulation of enteric neuromuscular function: implications for motility and inflammatory disorders. Gastroenterology. 1996;111:1683-1699. [Cited in This Article: ] |
| 17. | Geboes K, Collins S. Structural abnormalities of the nervous system in Crohn’s disease and ulcerative colitis. Neurogastroenterol Motil. 1998;10:189-202. [Cited in This Article: ] |
| 18. | Vermillion DL, Huizinga JD, Riddell RH, Collins SM. Altered small intestinal smooth muscle function in Crohn’s disease. Gastroenterology. 1993;104:1692-1699. [Cited in This Article: ] |
| 19. | Vrees MD, Pricolo VE, Potenti FM, Cao W. Abnormal motility in patients with ulcerative colitis: the role of inflammatory cytokines. Arch Surg. 2002;137:439-445; discussion 445-446. [Cited in This Article: ] |
| 20. | Reddy SN, Bazzocchi G, Chan S, Akashi K, Villanueva-Meyer J, Yanni G, Mena I, Snape WJ Jr. Colonic motility and transit in health and ulcerative colitis. Gastroenterology. 1991;101:1289-1297. [Cited in This Article: ] |
| 21. | Kristinsson JO, Hopman WP, Oyen WJ, Drenth JP. Gastroparesis in patients with inactive Crohn’s disease: a case series. BMC Gastroenterol. 2007;7:11. [Cited in This Article: ] |
| 22. | Castiglione F, Del Vecchio Blanco G, Rispo A, Petrelli G, Amalfi G, Cozzolino A, Cuccaro I, Mazzacca G. Orocecal transit time and bacterial overgrowth in patients with Crohn’s disease. J Clin Gastroenterol. 2000;31:63-66. [Cited in This Article: ] |
| 23. | Annese V, Bassotti G, Napolitano G, Frusciante V, Bruno M, Conoscitore P, Germani U, Morelli A, Andriulli A. Gastric emptying of solids in patients with nonobstructive Crohn’s disease is sometimes delayed. J Clin Gastroenterol. 1995;21:279-282. [Cited in This Article: ] |
| 24. | Kohno N, Nomura M, Okamoto H, Kaji M, Ito S. The use of electrogastrography and external ultrasonography to evaluate gastric motility in Crohn’s disease. J Med Invest. 2006;53:277-284. [Cited in This Article: ] |
| 25. | Moreels TG, De Man JG, De Winter BY, Timmermans JP, Herman AG, Pelckmans PA. Effect of 2,4,6-trinitrobenzenesulphonic acid (TNBS)-induced ileitis on the motor function of non-inflamed rat gastric fundus. Neurogastroenterol Motil. 2001;13:339-352. [Cited in This Article: ] |
| 26. | Neurath MF, Fuss I, Kelsall BL, Stüber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1281-1290. [Cited in This Article: ] |
| 27. | Ruyssers NE, De Winter BY, De Man JG, Loukas A, Pearson MS, Weinstock JV, Van den Bossche RM, Martinet W, Pelckmans PA, Moreels TG. Therapeutic potential of helminth soluble proteins in TNBS-induced colitis in mice. Inflamm Bowel Dis. 2009;15:491-500. [Cited in This Article: ] |
| 28. | Menachem Y, Trop S, Kolker O, Shibolet O, Alper R, Nagler A, Ilan Y. Adoptive transfer of NK 1.1+ lymphocytes in immune-mediated colitis: a pro-inflammatory or a tolerizing subgroup of cells? Microbes Infect. 2005;7:825-835. [Cited in This Article: ] |
| 29. | Moreels TG, De Man JG, Bogers JJ, De Winter BY, Vrolix G, Herman AG, Van Marck EA, Pelckmans PA. Effect of Schistosoma mansoni-induced granulomatous inflammation on murine gastrointestinal motility. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1030-G1042. [Cited in This Article: ] |
| 30. | Seerden TC, De Winter BY, Van Den Bossche RM, Herman AG, Pelckmans PA, De Man JG. Regional differences in gastrointestinal motility disturbances during acute necrotising pancreatitis. Neurogastroenterol Motil. 2005;17:671-679. [Cited in This Article: ] |
| 31. | Seerden TC, De Man JG, Holzer P, Van den Bossche RM, Herman AG, Pelckmans PA, De Winter BY. Experimental pancreatitis disturbs gastrointestinal and colonic motility in mice: effect of the prokinetic agent tegaserod. Neurogastroenterol Motil. 2007;19:856-864. [Cited in This Article: ] |
| 32. | Mashimo H, Kjellin A, Goyal RK. Gastric stasis in neuronal nitric oxide synthase-deficient knockout mice. Gastroenterology. 2000;119:766-773. [Cited in This Article: ] |
| 33. | Setiawan T, Metwali A, Blum AM, Ince MN, Urban JF Jr, Elliott DE, Weinstock JV. Heligmosomoides polygyrus promotes regulatory T-cell cytokine production in the murine normal distal intestine. Infect Immun. 2007;75:4655-4663. [Cited in This Article: ] |
| 34. | Weinstock JV, Summers RW, Elliott DE. Role of helminths in regulating mucosal inflammation. Springer Semin Immunopathol. 2005;27:249-271. [Cited in This Article: ] |
| 35. | De Schepper HU, De Man JG, Van Nassauw L, Timmermans JP, Herman AG, Pelckmans PA, De Winter BY. Acute distal colitis impairs gastric emptying in rats via an extrinsic neuronal reflex pathway involving the pelvic nerve. Gut. 2007;56:195-202. [Cited in This Article: ] |
| 36. | Khan WI, Collins SM. Immune-mediated alteration in gut physiology and its role in host defence in nematode infection. Parasite Immunol. 2004;26:319-326. [Cited in This Article: ] |
| 37. | Khan WI, Collins SM. Gut motor function: immunological control in enteric infection and inflammation. Clin Exp Immunol. 2006;143:389-397. [Cited in This Article: ] |
| 38. | Moreels TG, De Man JG, De Winter BY, Herman AG, Pelckmans PA. Effect of Interleukin-1beta on cholinergic contractions in the gastrointestinal tract of the rat. Anaesthesiol Intensivmed Notfallmed Schmerzther. 1998;33:610. [Cited in This Article: ] |
| 39. | Akiho H, Blennerhassett P, Deng Y, Collins SM. Role of IL-4, IL-13, and STAT6 in inflammation-induced hypercontractility of murine smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2002;282:G226-G232. [Cited in This Article: ] |
| 40. | Stoffels B, Schmidt J, Nakao A, Nazir A, Chanthaphavong RS, Bauer AJ. Role of interleukin 10 in murine postoperative ileus. Gut. 2009;58:648-660. [Cited in This Article: ] |
| 41. | Zhang Z, Hinrichs DJ, Lu H, Chen H, Zhong W, Kolls JK. After interleukin-12p40, are interleukin-23 and interleukin-17 the next therapeutic targets for inflammatory bowel disease? Int Immunopharmacol. 2007;7:409-416. [Cited in This Article: ] |
| 42. | Elliott DE, Summers RW, Weinstock JV. Helminths and the modulation of mucosal inflammation. Curr Opin Gastroenterol. 2005;21:51-58. [Cited in This Article: ] |
| 43. | Smith P, Mangan NE, Walsh CM, Fallon RE, McKenzie AN, van Rooijen N, Fallon PG. Infection with a helminth parasite prevents experimental colitis via a macrophage-mediated mechanism. J Immunol. 2007;178:4557-4566. [Cited in This Article: ] |
| 44. | Kane CM, Cervi L, Sun J, McKee AS, Masek KS, Shapira S, Hunter CA, Pearce EJ. Helminth antigens modulate TLR-initiated dendritic cell activation. J Immunol. 2004;173:7454-7461. [Cited in This Article: ] |
| 45. | Niess JH, Reinecker HC. Dendritic cells: the commanders-in-chief of mucosal immune defenses. Curr Opin Gastroenterol. 2006;22:354-360. [Cited in This Article: ] |