Copyright
©2010 Baishideng Publishing Group Co.
World J Gastroenterol. Dec 21, 2010; 16(47): 5925-5935
Published online Dec 21, 2010. doi: 10.3748/wjg.v16.i47.5925
Published online Dec 21, 2010. doi: 10.3748/wjg.v16.i47.5925
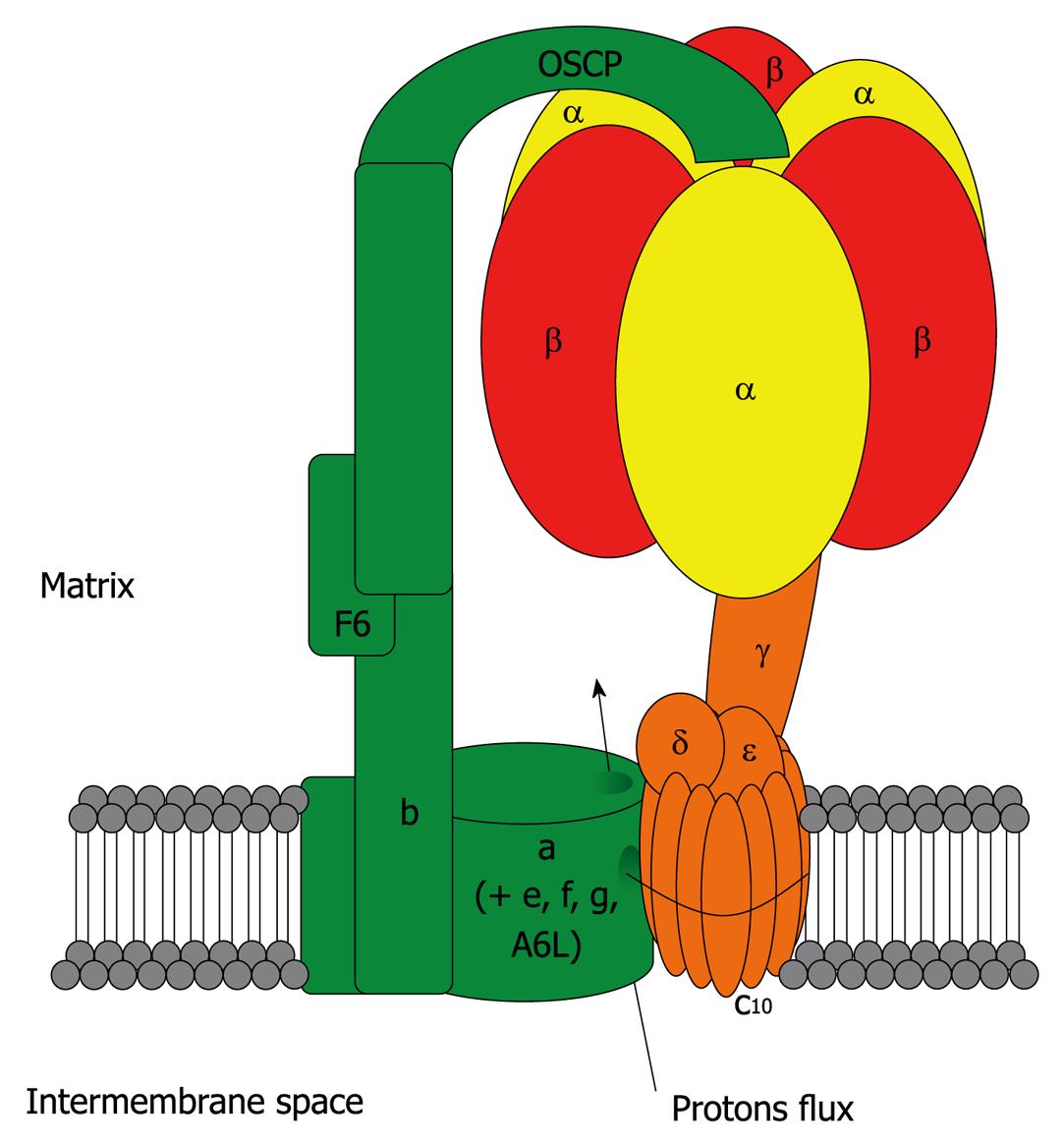
Figure 1 Mitochondrial ATP synthase.
In eukaryotic cells, F1FO ATP synthase is a complex molecular motor composed of 16 unique subunits (α, β and c being present in multiple copies in the whole complex). It comprises the activity-bearing subunits (three αβ heterodimers), a rotor (in orange: subunits γ, δ, ε and the c ring) and a stator (in green: subunits a, e, f, g, A6L, b, F6, d and OSCP) which holds the αβ heterodimers. It is organized into two major domains: a soluble F1 domain (α, β, γ, δ, ε) and a membrane-associated FO domain. The F1 domain bears the catalytic activity while the FO domain is a proton channel. The FO domain uses the proton gradient created by the four other complexes of the respiratory chain to drive the rotation of the central stalk, which will alternatively change the conformation of the three αβ heterodimers, leading to the synthesis of ATP from ADP and Pi. In the case of the eukaryotic ATP synthase, the translocation of 10 protons through the c ring will lead to a full turn, and the synthesis of 3 ATP molecules. In the absence of a proton gradient, or if the F1 domain is isolated from the FO domain, the enzyme behaves as an ATP hydrolase.
- Citation: Vantourout P, Radojkovic C, Lichtenstein L, Pons V, Champagne E, Martinez LO. Ecto-F1-ATPase: A moonlighting protein complex and an unexpected apoA-I receptor. World J Gastroenterol 2010; 16(47): 5925-5935
- URL: https://www.wjgnet.com/1007-9327/full/v16/i47/5925.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i47.5925