Published online Oct 7, 2010. doi: 10.3748/wjg.v16.i37.4709
Revised: May 20, 2010
Accepted: May 27, 2010
Published online: October 7, 2010
AIM: To investigate the usefulness of magnified observations of iodine-unstained esophageal lesions in the histological diagnosis of esophageal mucosa abnormalities, in high-risk esophageal cancer groups.
METHODS: The subjects included 38 patients who had at least one of the four criteria known to be high-risk factors for esophageal cancer. Following endoscopic observation, magnified observations were performed on iodine-unstained lesions of the esophagus. The total number of lesions was 43. These lesions were classified as type A (clear papilla), type B (fused papilla), and type C (non-visible papilla) according to the findings. Tissue biopsy was then carried out. Finally the histological findings were graded in terms of histological factors, and their relationships were compared.
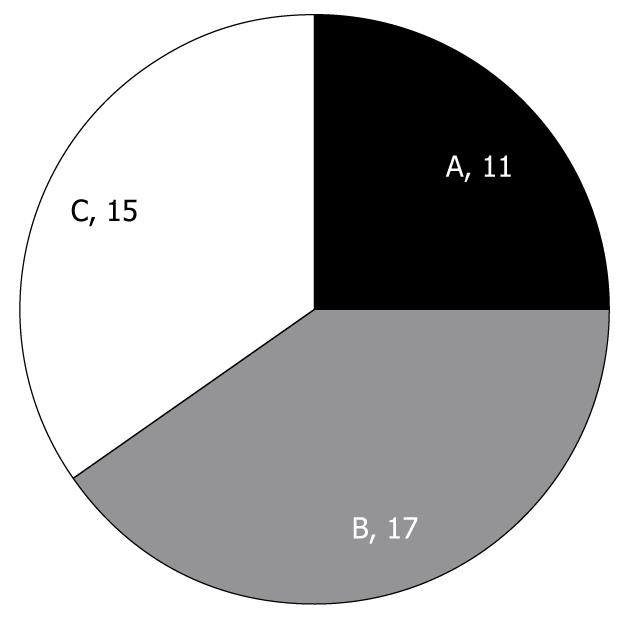
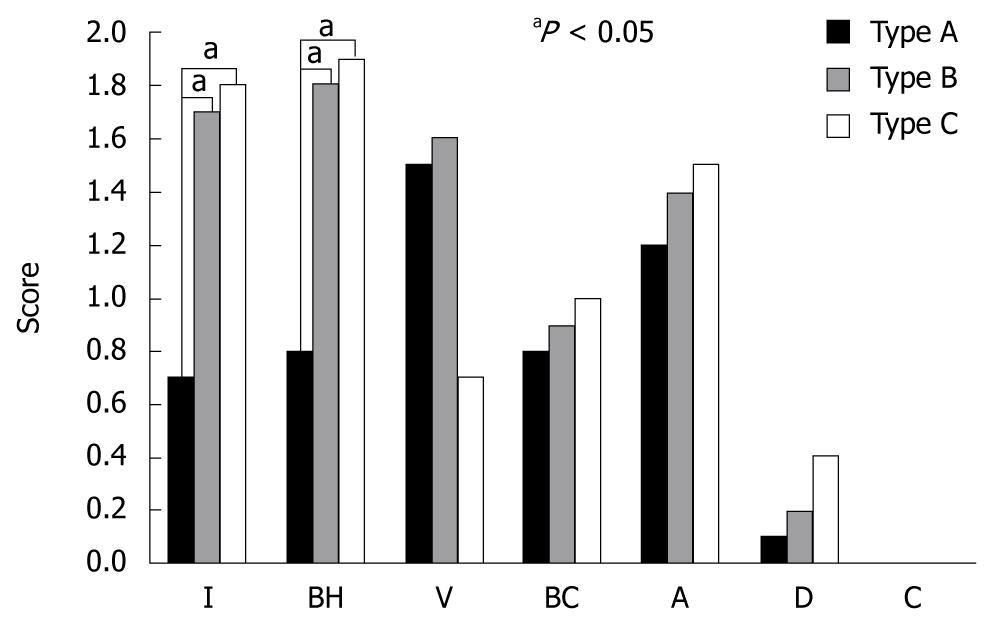
RESULTS: Of the 43 lesions, 11 were type A, 17 were type B, and 15 were type C under magnifying endoscopy. Histological findings such as inflammatory cell infiltration and basal cell hyperplasia were significantly increased in type B and type C lesions compared with type A lesions (P < 0.05). Low-grade esophageal dysplasia was apparent in 1 (9%) of 11 type A lesions, in 3 (18%) of 17 type B lesions, and in 6 (40%) of 15 type C lesions, with the highest rate in type C.
CONCLUSION: Magnified observations of the esophagus, classified by papillary aspects using magnifying endoscopy of iodine-unstained lesions in high-risk esophageal cancer groups, are considered useful in estimating dysplasia and inflammation of esophageal mucosa.
- Citation: Choi IS, Jang JY, Cho WY, Lee TH, Kim HG, Lee BY, Jeong SW, Cho JY, Lee JS, Jin SY. Usefulness of magnifying endoscopy for iodine-unstained lesions in a high-risk esophageal cancer population. World J Gastroenterol 2010; 16(37): 4709-4715
- URL: https://www.wjgnet.com/1007-9327/full/v16/i37/4709.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i37.4709
Although early diagnosis of esophageal cancer is known to be an important determinant of clinical outcome, it is not easy to diagnose early-stage esophageal cancer with conventional endoscopy[1]. The best complementary measure for this is the iodine staining technique, which is used for the diagnosis of early-stage esophageal cancer, especially in high-risk esophageal cancer groups. However, the iodine staining technique has low specificity in diagnosing esophageal cancer[2], and therefore, the development of other diagnostic measures are needed to complement this technique. The recent development of magnifying endoscopy has enabled more detailed observations of various gastrointestinal disorders. In addition, magnified observations of the aspects of small blood vessels and microscopic surface structure has been proved to be clinically useful, however, the clinical application of magnifying endoscopy is at an early stage. The present study was performed to determine the usefulness of magnified observations of iodine-unstained esophageal lesions in the histological diagnosis of esophageal mucosa abnormalities.
The subjects included in this study had at least one of the four criteria known as high-risk factors for esophageal cancer, which were older age, smokers, alcoholics or those with a history of non-esophageal primary malignant tumor. The cut-off points for old age, smoking and drinking were 55 years and 5 d/wk, respectively. Patients were excluded if they had dysphagia, recent upper gastrointestinal hemorrhage, known liver cirrhosis or cardiac or coagulation disorders (Table 1).
| Inclusion criteria | Exclusion criteria |
| Old age (> 55 yr old) | Dysphagia |
| Smoking (> 5 d/wk) | Recent upper GI hemorrhage |
| Alcohol intake (> 5 d/wk) | Liver cirrhosis |
| History of non-esophageal primary cancer | Cardiac or coagulation disorder |
Thirty eight subjects were included. Multiple iodine-unstained lesions were observed in 7 patients, and 3 patients had no iodine-unstained lesions. The total number of lesions was 43. The average age of these patients was 61.4 years, the male to female ratio was 24:14, 14 of 38 patients smoked, with an average smoking history of 29.4 packs/year, and 10 of 38 patients consumed alcohol, with an average consumption of 367 g/wk. In addition, 11 patients had a history of non-esophageal primary malignant tumors, including gastric cancer in 10 patients, and breast cancer in 1 patient (Table 2).
| Age (yr) | 61.4 (43-86) |
| Sex (M:F) | 24:4 |
| Smoking amount (packs/yr) | 29.4 (14 patients) |
| Alcohol intake dose (g/wk) | 367 (6 patients) |
| History of non-esophageal carcinoma | Stomach cancer: 10, Breast cancer: 1 |
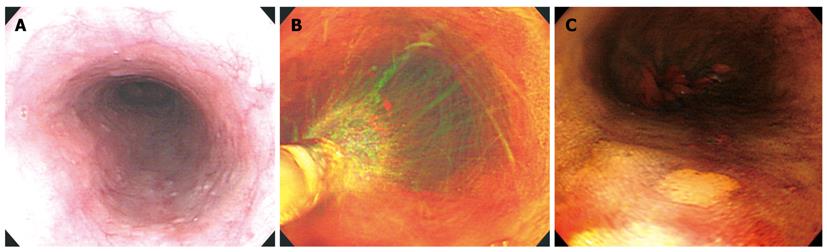
Following conventional endoscopic inspection in patients who were classified in the high-risk esophageal cancer group, mucus was removed by spraying water onto the entire esophagus. A polyethylene catheter was passed down through the biopsy channel and 20 mL of 1.5% iodine solution was sprayed on the mucosal surface, followed by identification of the presence of iodine-unstained lesions. The size of the iodine-unstained lesions was measured at the time of detection, to include lesions over 3 mm and less than 30 mm. Magnified observations were then performed (Figure 1).
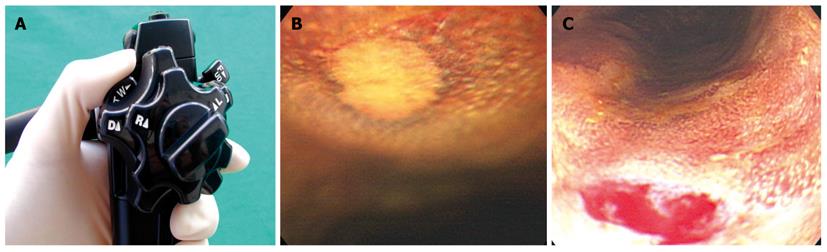
The magnifying endoscope used in this study was a GIF-Q240Z (Olympus Co., Ltd, Tokyo, Japan) with a maximum magnification power of 80 ×. When the tip of the endoscope approached the target area, the zoom lever was pulled inferiorly as shown in Figure 2A, B, and 80 × magnification observations were performed. Following magnified observations on the iodine-unstained lesions, tissue biopsy was performed in the same area (Figure 2).
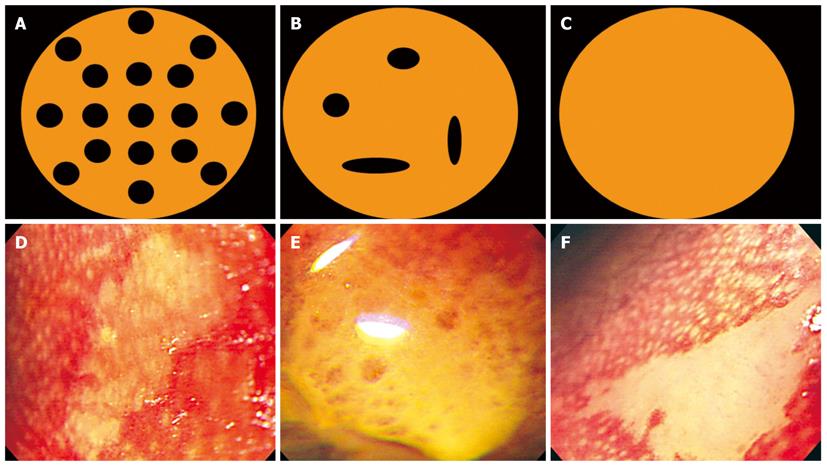
The magnified observations on each of the iodine-unstained lesions were classified into the following three categories: clear papilla with well-maintained and regularly arranged papillae was classified as type A; fused papilla in which papillae could be seen but were not regular and were either merged or partially seen was classified as type B; and non-visible papilla in which papillae were not observed at all was classified as type C. This reflected the classification of non-iodine stained lesions outlined by Arima et al[3] in Japan. In addition, photographs and video were taken of these lesions in order to reduce the interobserver variation, and were determined by two specialists in endoscopy who did not participate in the examination. The views of at least 2 of the 3 specialists were compared to that of the examiner, and all were found to be consistent in the classification of each mucosal form (Figure 3).
Following magnified observations of the iodine-unstained lesions, and their classification which was followed by tissue biopsy in the same area, the tissue was graded according to the following histological factors: inflammatory cell infiltration, basal cell hyperplasia, vascular lake, balloon cell, acanthosis, dysplasia, carcinoma grade from 0 to 3 according to their extent (0: normal, 1: mild, 2: moderate, 3: high) and analysis of their correlation with the types found on magnifying endoscopy.
Statistical data are described as the mean ± SD, and one-way ANOVA and Chi-square were used for the analysis. Data were significant when P < 0.05.
According to papillary form, magnified observations of iodine-unstained lesions showed 11 lesions of type A, 17 lesions of type B and 15 lesions of type C, with type B being the most frequent (Figure 4). When examining age, smoking history, alcohol consumption, presence of non-esophageal tumor, and the size of the iodine-unstained area based on the type of papillary form, the average age was 59.8 ± 5.1 years for type A, 65.8 ± 11.6 years for type B, and 55.8 ± 8.8 years for type C; alcohol consumption was 106.9 ± 222.1 g/wk for type A, 67.7 ± 123.3 g/wk for type B, and 162.5 ± 251.9 g/wk for type C; smoking history was 12.8 ± 17.5 packs/year for type A, 13.8 ± 19.6 packs/year for type B, and 7.5 ± 10.6 packs/year for type C; non-esophageal tumor was found in 2/11 cases with type A, 4/17 cases with type B, and 5/15 cases with type C; and the size of the iodine-unstained area was 7.0 ± 4.8 mm for type A, 9.4 ± 7.5 mm for type B, and 5.7 ± 1.8 mm for type C, and thus did not show any significant difference between the different types.
The findings on magnifying endoscopy i.e., the histological findings based on the papillary form showed that the total score for each histological factor increased as it moved from type A to type B, and type C (5.1 ± 2.4 for type A, 7.4 ± 2.7 for type B, and 7.4 ± 2.9 for type C). In particular, inflammatory cell infiltration and basal cell hyperplasia associated with the degree of inflammation in the histological findings was significantly increased in type B and C compared with type A (P < 0.05) (Figure 5). Low-grade esophageal dysplasia was apparent in 1 of 11 type A lesions, in 3 (21%) of 17 type B lesions, and in 6 (44%) of 15 type C lesions, with type C showing the highest rate with no statistical significance, however, dysplasia showed a tendency to increase from type A to type B and type C (Table 3). There was no high-grade dysplasia or carcinoma in any of the lesion types. When examining age, smoking history, alcohol consumption, presence of non-esophageal tumor, and the size of the iodine-unstained area based on the presence of dysplasia, the average age was 63.0 ± 9.7 years in the non-dysplasia group, and 56.1 ± 10.8 years in the dysplasia group; alcohol consumption was 135.9 ± 207.0 g/wk in the non-dysplasia group, and 122.5 ± 245.0 g/wk in the dysplasia group; smoking history was 14.1 ± 18.0 packs/year in the non-dysplasia group, and 3.8 ± 7.4 packs/year in the dysplasia group; non-esophageal tumors were found in 10/33 cases in the non-dysplasia group, and in 1/10 cases in the dysplasia group; the size of the iodine-unstained lesion was 6.8 ± 4.0 mm in the non-dysplasia group, and 9.5 ± 8.6 mm in the dysplasia group. No significant differences were found between patients with and without dysplasia.
| Type A | Type B | Type C | |
| Dysplasia (-) | 10 | 14 | 9 |
| Dysplasia (+) | 1 | 3 | 6 |
| Dysplasia/total | 1/11 (9%) | 3/17 (18%) | 6/15 (40%) |
While most patients with symptoms caused by squamous cell carcinoma of the esophagus have a poor prognosis, due to an advanced stage at the time of diagnosis, the 5-year survival rate of superficial esophageal cancer, in which the area of invasion does not include the submucosal layer, is very high at 70%-80%. In particular, esophageal cancers limited to the epithelium or lamina propria have a low risk of lymph node metastasis, and thus successful treatment is possible with endoscopic mucosal resection. As such, the 5-year survival rate is known to be over 95%. Therefore, early diagnosis of esophageal cancer is an important determinant of clinical outcome. However, it is very difficult to diagnose early-stage esophageal cancer with conventional endoscopic and radiologic examinations[1]. Lugol’s solution, which is used for chromoscopy of the esophagus, is an absorbent dye based on iodine which has an affinity for glycogen in non-keratinized squamous epithelium. This solution turns a dark greenish-brown color, and gradually becomes lighter with time. It is diluted to 1%-5% when used, and is applied using a catheter. There is no glycogen present in inflammatory squamous epithelia such as erosive esophagitis, neoplastic tissue, dysplasia or non-squamous epithelium such as columnar epithelium. Therefore, these lesions remain unstained, while glycogenic acanthosis is stained darker. Such iodine staining of the esophagus allows the early diagnosis of esophageal cancer compared with conventional endoscopy. This technique also enables a more definite pre-operative diagnosis of the range of esophageal cancers[4,5]. However, while chromoscopy of the esophagus using iodine has few false-negative results and has high sensitivity, staining cannot be performed in diseases other than esophageal cancer. Therefore, it is limited in that it is not specific to early-stage esophageal cancer[2]. Consequently, the development of more effective methods of examination is needed in addition to histological diagnosis. Squamous cell carcinoma of the esophagus has wide regional differences and has a very high prevalence in China and Iran, but is rare in Japan, America and Europe. Older age, smoking, alcohol consumption and insufficient intake of fresh fruit and vegetables are known to be risk factors, and it has recently been reported that the prevalence of multiple primary tumors is increasing[1]. Kodama et al[6] reported that non-esophageal primary carcinoma was present in 20.6% of 2418 superficial esophageal cancer patients, and Shimizu et al[1] reported that non-esophageal primary carcinoma was present in 29.2% of 233 patients who received treatment with either surgery or endoscopic mucosal resection and were followed up. In particular, head and neck tumors are known to be high risk factors for causing esophageal cancer. Although there are no definite standards for age, smoking and alcohol consumption, advanced age, smoking, and alcohol consumption are high risk factors for esophageal cancer[1,7]. Considering the cost-effectiveness of iodine staining and patient discomfort, the preselection of high-risk patients seems to be a more cost-effective procedure during screening examinations. Therefore, the present study selected subjects based on the above-mentioned standards. Magnifying endoscopy of the digestive tract observes the mucosal forms of the digestive tract in detail, using an endoscope with a magnifying power of over 30 ×[8]. The origins of endoscopy was based on the observations of foveola in the stomach by Gutzeit et al[9] in 1954 and on observations by Takemoto et al[10] in 1966 in Japan. Sakaki et al[11] presented the first classification of gastric mucosa on magnifying endoscopy, especially atrophic gastritis, by dividing mucosa into 5 different types. Focal adjustment is difficult in the esophagus due to peristaltic movement, respiration, and heart beat, whereas the advantages in the large intestines are, that it is histologically uniform, there is no chronic inflammation, and there is almost no difference in the normal pit patterns between different individuals. Thanks to these advantages, magnified observations are more useful in the lower digestive tract than in the upper digestive tract, and although its use in the lower digestive tract was started later, it has advanced rapidly. It is no exaggeration to say that the work of Kudo et al[12] on magnifying endoscopy of the large intestine has resulted in an increase in current magnified observations. However, as the high-pixel electronic endoscope has recently been used more generally, various studies are being carried out not only on the large intestine, but also in the esophagus and the stomach. Magnifying endoscopy is useful in the differential diagnosis of non-tumor and tumor through the observation of papillary blood vessels in the esophagus, and its range of use is expanding to the stomach to determine the range of mucosal resection for early-stage stomach cancer or the presence of recurrence after endoscopic therapy[13]. The present study employed magnifying endoscopy to complement the low specificity of iodine staining in the esophagus. In 1997, Arima et al[3] performed magnifying endoscopy on esophageal mucosa from resected specimens of esophageal cancer. A total of 55 unstained lesions less than 3 cm in diameter from 22 patients were studied. Similarly, 114 unstained lesions were studied in vivo using magnifying endoscopy and were classified according to papillary pattern. The findings in both groups were compared with histological findings and showed a favorable co-relationship. The size of unstained lesions was limited to between 3 mm and 30 mm, because carcinoma is known to be extremely rare in lesions less than 3 mm, whereas most carcinomas occur in lesions over 30 mm. The classification of types and size used in the current study was also identical to these authors, and although their results were different from those observed in this study, the findings showed an increase in the frequency of dysplasia and an increase in inflammatory cell infiltration from type A to type C similar to our findings. Antonioli[14] reported that approximately 30% of patients with severe esophageal dysplasia progressed to invasive carcinoma. This frequency was 15% for mild dysplasia. Thus, dysplasia seems to be a precursor of squamous cell carcinoma in the upper digestive mucosa and has a direct correlation with severity of cellular abnormalities and progression to invasive carcinoma. Rubio et al[15] classified mild and moderate dysplasia as low-grade intraepithelial carcinoma and severe dysplasia as high-grade carcinoma. Magnifying endoscopic observations of the esophagus did not have a significant diagnostic role relative to the pit pattern of gastric or colonic mucosa, as the former was covered with squamous epithelium and thus had a smooth amorphous surface[16].
Methylene blue, indigo carmine, or acetic acid chromoendoscopy combined with magnification endoscopy also allows identification of specific mucosal patterns (tubular, ridged, or villous) which are highly associated with the presence of specialized intestinal metaplasia in patients with Barrett’s esophagus in directed biopsy examinations.[17]. Two prospective Japanese cohort studies[18,19] reported high specificity (92%-100%), but sensitivity was variable (53%-85%).
However, as a result of the leading studies on magnification observations of esophageal mucosa by Arima et al[3,20] and Inoue et al [21-23], diagnostic standards have been established to some extent. Arima et al[20] classified 4 different types through the enhanced findings of papillary blood vessels, other than the 3 types mentioned above, in a study on the magnifying endoscopic diagnosis of superficial esophageal cancer. These authors reported that the risk of esophageal cancer was high and the depth of cancer invasion was also high since these papillary blood vessels showed irregular shapes or were dilated. In addition, in a study on magnifying endoscopic observations with the highest magnification power, Inoue et al[21-23] reported that the intrapapillary capillary network, or the thickness of neoplastic blood vessels, increased with progression of the depth of cancer invasion in addition to the shape becoming irregular. By doing so, they stated that magnifying endoscopic diagnosis of the depth of invasion of esophageal cancer was possible and reported that ultrasonic endoscopy was outstanding in the diagnosis of elevated lesions. However, magnifying endoscopic diagnosis was also useful for the diagnosis of depressed lesions. As shown in our study, an increase in the frequency of dysplasia and an increase in inflammation from type A to type C were observed in the high-risk esophageal cancer group. Enhanced findings of the esophagus, through classification of the papillary forms, using magnifying endoscopy on iodine-unstained lesions, seems useful in the diagnosis of dysplasia and inflammation of esophageal mucosa. More case studies on magnifying endoscopy should be performed in the future.
Various chromoendoscopy techniques combined with magnifying endoscopy are useful in assessing the presence of specialized intestinal metaplasia in patients with Barrett’s esophagus. However, little data is currently available regarding the clinical usefulness of magnifying endoscopy using Lugol’s solution in a high-risk esophageal cancer population.
In this study, the authors demonstrate the clinical usefulness of magnifying endoscopy for iodine-unstained lesions in a high-risk esophageal cancer population.
This study demonstrates that magnified observations of the esophagus classified by papillary aspects using magnifying endoscopy on iodine-unstained lesions in a high-risk esophageal cancer group are useful in estimating dysplasia and inflammation of esophageal mucosa.
By providing an understanding of the relationships between magnified observations on iodine unstained lesions and esophageal mucosal dysplasia and inflammation, the results of this study may represent a future strategy in the management of patients with a high risk of esophageal cancer.
The magnified observations on each of the iodine-unstained lesions were classified into the following three categories: type A, clear papilla with well maintained and regularly arranged papillae; type B, fused papilla in which papillae could be seen but were not regular and were either merged or partially seen; type C, non-visible papilla in which papillae were not observed at all.
This is a useful study on the subject of dye spray to facilitate detection of dysplasia. In the West adenocarcinoma is much more common and although not the main focus of this study, some reference should be made to dye spray in Barretts.
Peer reviewer: William Dickey, Professor, Altnagelvin Hospital, Londonderry, BT47 6SB, Northern Ireland, United Kingdom
S- Editor Wang YR L- Editor Webster JR E- Editor Ma WH
| 1. | Shimizu Y, Tukagoshi H, Fujita M, Hosokawa M, Kato M, Asaka M. Endoscopic screening for early esophageal cancer by iodine staining in patients with other current or prior primary cancers. Gastrointest Endosc. 2001;53:1-5. [Cited in This Article: ] |
| 2. | Tincani AJ, Brandalise N, Altemani A, Scanavini RC, Valério JB, Lage HT, Molina G, Martins AS. Diagnosis of superficial esophageal cancer and dysplasia using endoscopic screening with a 2% lugol dye solution in patients with head and neck cancer. Head Neck. 2000;22:170-174. [Cited in This Article: ] |
| 3. | Arima H, Arima M, Kouzu T. Magnified observation of non-iodine staining lesion of the esophagus. Gastroenterol Endosc. 1997;39:1557-1565. [Cited in This Article: ] |
| 4. | Shim CS. Staining in gastrointestinal endoscopy: clinical applications and limitations. Endoscopy. 1999;31:487-496. [Cited in This Article: ] |
| 5. | Canto MI. Vital staining and Barrett’s esophagus. Gastrointest Endosc. 1999;49:S12-S16. [Cited in This Article: ] |
| 6. | Kodama M, Kakegawa T. Treatment of superficial cancer of the esophagus: a summary of responses to a questionnaire on superficial cancer of the esophagus in Japan. Surgery. 1998;123:432-439. [Cited in This Article: ] |
| 7. | Meyer V, Burtin P, Bour B, Blanchi A, Cales P, Oberti F, Person B, Croue A, Dohn S, Benoit R. Endoscopic detection of early esophageal cancer in a high-risk population: does Lugol staining improve videoendoscopy? Gastrointest Endosc. 1997;45:480-484. [Cited in This Article: ] |
| 8. | Tanaka M. What is magnifying endoscopy? Gastroenterol Endosc. 2001;13:286-292. [Cited in This Article: ] |
| 9. | Gutzeit K, Teitge U. Die Gastroskopie. Lehrbach und Atlas. Müchen: Urban & Schwachenberg 1954; . [Cited in This Article: ] |
| 10. | Takemoto T. Endoscopic diagnosis of chronic gastritis. J Diagn Treatm. 1966;54:1274. [Cited in This Article: ] |
| 11. | Sakaki N, Iida Y, Okazaki Y, Kawamura S, Takemoto T. Magnifying endoscopic observation of the gastric mucosa, particularly in patients with atrophic gastritis. Endoscopy. 1978;10:269-274. [Cited in This Article: ] |
| 12. | Kudo S, Hirota S, Nakajima T, Hosobe S, Kusaka H, Kobayashi T, Himori M, Yagyuu A. Colorectal tumours and pit pattern. J Clin Pathol. 1994;47:880-885. [Cited in This Article: ] |
| 13. | Kusaka T, Fujimori T, Chiba T. Magnifying endoscopy: Its purpose and usefulness. Gastroenterol Endosc. 2001;13:293-300. [Cited in This Article: ] |
| 14. | Antonioli D. Esophagus. Pathology of incipient neoplasia. 2nd ed. Philadelphia: W.B. Saunders Company 1993; 64-71. [Cited in This Article: ] |
| 15. | Rubio CA, Liu FS, Zhao HZ. Histological classification of intraepithelial neoplasias and microinvasive squamous carcinoma of the esophagus. Am J Surg Pathol. 1989;13:685-690. [Cited in This Article: ] |
| 16. | Kumagai Y, Inoue H, Nagai K, Kawano T, Iwai T. Magnifying endoscopy, stereoscopic microscopy, and the microvascular architecture of superficial esophageal carcinoma. Endoscopy. 2002;34:369-375. [Cited in This Article: ] |
| 17. | Canto MI. Chromoendoscopy and magnifying endoscopy for Barrett's esophagus. Clin Gastroenterol Hepatol. 2005;3:S12-S15. [Cited in This Article: ] |
| 18. | Yagi K, Nakamura A, Sekine A. Accuracy of magnifying endoscopy with methylene blue in the diagnosis of specialized intestinal metaplasia and short-segment Barrett’s esophagus in Japanese patients without Helicobacter pylori infection. Gastrointest Endosc. 2003;58:189-195. [Cited in This Article: ] |
| 19. | Endo T, Awakawa T, Takahashi H, Arimura Y, Itoh F, Yamashita K, Sasaki S, Yamamoto H, Tang X, Imai K. Classification of Barrett’s epithelium by magnifying endoscopy. Gastrointest Endosc. 2002;55:641-647. [Cited in This Article: ] |
| 20. | Arima M. Magnifying endoscopy for diagnosis of superficial esophageal cancer. Gastroenterol Endosc. 2001;13:309-318. [Cited in This Article: ] |
| 21. | Inoue1 H, Honda T, Nagai K, Kawano T, Yoshino K, Takeshita K, Endo M. Ultra-high magnification endoscopic observation of carcinoma in situ of the esophagus. Dig Endosc. 1996;8:134-138. [Cited in This Article: ] |
| 22. | Inoue H, Honda T, Nagai K, Kawano T, Yoshino K, Takeshita K, Endo M. Ultra-high magnification endoscopic observation of carcinoma in situ of the esophagus. Dig Endosc. 1997;9:16-18. [Cited in This Article: ] |
| 23. | Inoue H, Kumagai Y, Yoshida T. Diagnosis of superficial esophageal lesions by high-magnification endoscopy. Gastroenterol Endosc. 2001;13:301-308. [Cited in This Article: ] |