Copyright
©2010 Baishideng.
World J Gastroenterol. Aug 14, 2010; 16(30): 3731-3742
Published online Aug 14, 2010. doi: 10.3748/wjg.v16.i30.3731
Published online Aug 14, 2010. doi: 10.3748/wjg.v16.i30.3731
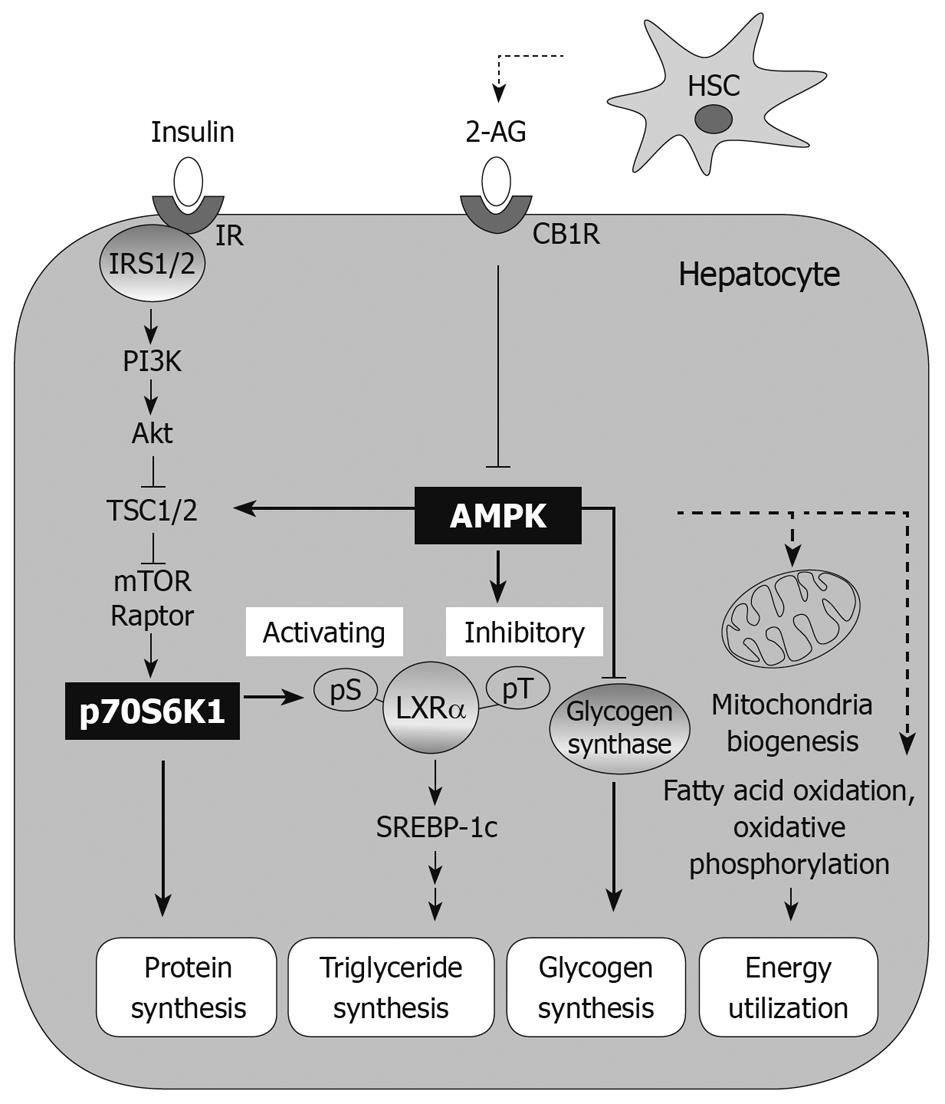
Figure 1 Adenosine monophosphate-activated protein kinase pathway in hepatic fuel metabolism.
Adenosine monophosphate-activated protein kinase (AMPK), a metabolic energy sensor, negatively regulates protein synthesis through inhibition of the mammalian target of rapamycin (mTOR)-S6 kinase-1 (S6K1) pathway. The inhibitory effect of AMPK on liver X receptor-α (LXRα)-dependent triglyceride synthesis is opposed by the action of S6K1. AMPK also shuts down glycogen synthesis via inhibitory phosphorylation of glycogen synthase. AMPK as a fuel sensor induces glucose transport and fat oxidation in response to metabolic stress such as energy deprivation, and also increases mitochondrial biogenesis. AMPK counteracts energy depletion by stimulating energy production and limiting energy utilization. Endocannabinoids such as 2-arachidonoylglycerol derived from hepatic stellate cells decrease AMPK phosphorylation resulting in downregulation of lipogenic action. 2-AG: 2-arachidonoylglycerol; CB1R: Cannabinoid 1 receptor; HSC: Hepatic stellate cell; IR: Insulin receptor; Raptor: Regulatory-associated protein of mTOR; IRS1: Insulin receptor substrate-1; PI3K: Phosphoinositide-3 kinase; TSC1: Tuberous sclerosis complex 1; pS: Phospho-serine; pT: Phospho-threonine; SREBP-1c: Sterol regulatory element binding protein-1c.
- Citation: Yang YM, Han CY, Kim YJ, Kim SG. AMPK-associated signaling to bridge the gap between fuel metabolism and hepatocyte viability. World J Gastroenterol 2010; 16(30): 3731-3742
- URL: https://www.wjgnet.com/1007-9327/full/v16/i30/3731.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i30.3731