Copyright
©2010 Baishideng.
World J Gastroenterol. Jul 7, 2010; 16(25): 3133-3143
Published online Jul 7, 2010. doi: 10.3748/wjg.v16.i25.3133
Published online Jul 7, 2010. doi: 10.3748/wjg.v16.i25.3133
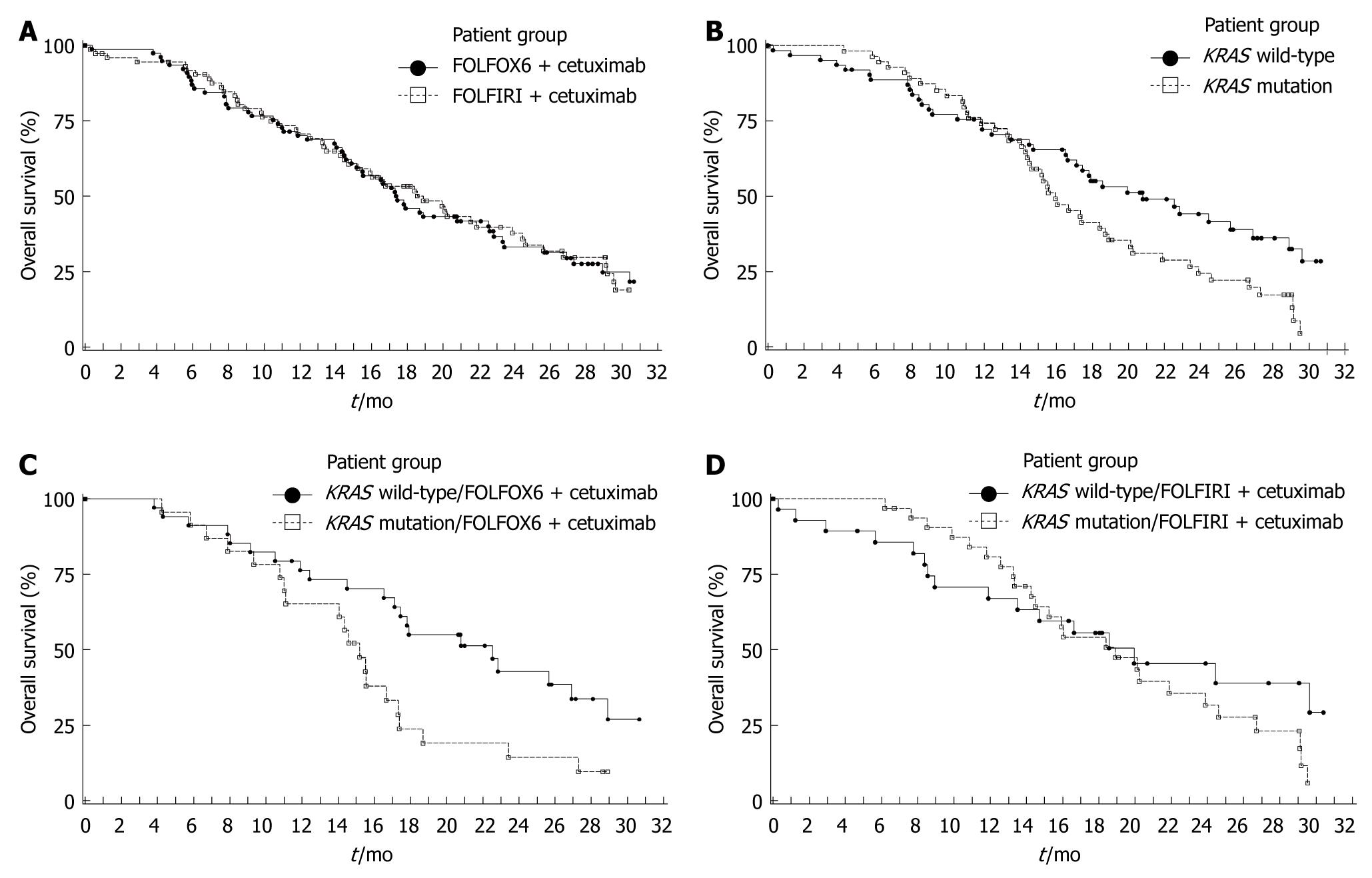
Figure 3 Kaplan Meier estimates for survival.
A: By treatment group in the ITT population, FOLFOX6 plus cetuximab (n = 77) vs FOLFIRI plus cetuximab (n = 74); B: By tumor KRAS mutation status, KRAS wild-type (n = 62) vs KRAS mutation (n = 55); C: By KRAS mutation status in patients receiving FOLFOX6 plus cetuximab, KRAS wild-type (n = 34) vs KRAS mutation (n = 23); D: By tumor KRAS mutation status in patients receiving FOLFIRI plus cetuximab, KRAS wild-type (n = 28) vs KRAS mutation (n = 32).
- Citation: Ocvirk J, Brodowicz T, Wrba F, Ciuleanu TE, Kurteva G, Beslija S, Koza I, Pápai Z, Messinger D, Yilmaz U, Faluhelyi Z, Yalcin S, Papamichael D, Wenczl M, Mrsic-Krmpotic Z, Shacham-Shmueli E, Vrbanec D, Esser R, Scheithauer W, Zielinski CC. Cetuximab plus FOLFOX6 or FOLFIRI in metastatic colorectal cancer: CECOG trial. World J Gastroenterol 2010; 16(25): 3133-3143
- URL: https://www.wjgnet.com/1007-9327/full/v16/i25/3133.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i25.3133