Copyright
©2010 Baishideng.
World J Gastroenterol. Jul 7, 2010; 16(25): 3133-3143
Published online Jul 7, 2010. doi: 10.3748/wjg.v16.i25.3133
Published online Jul 7, 2010. doi: 10.3748/wjg.v16.i25.3133
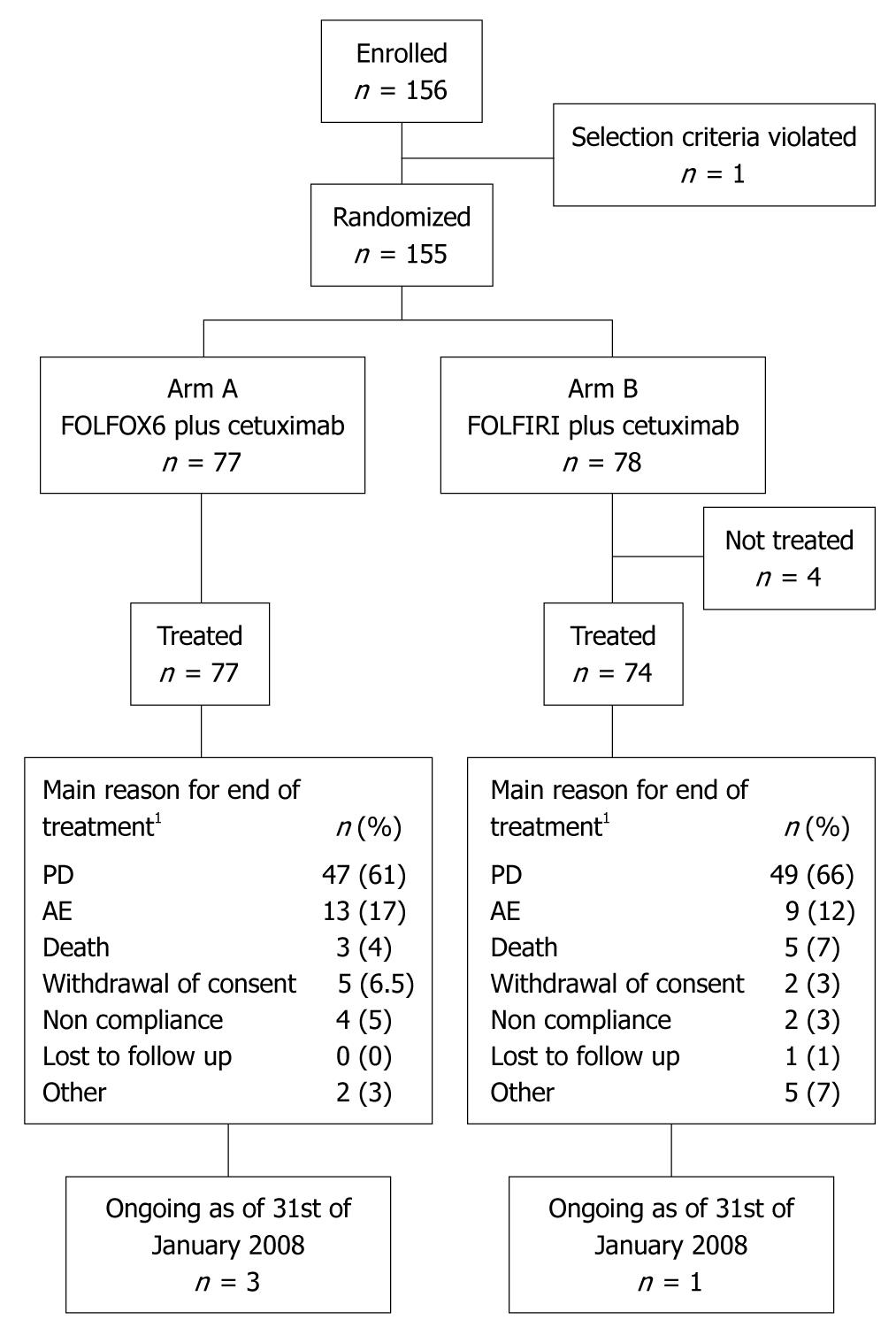
Figure 1 Disposition of patients as of clinical cut-off date January 2008.
The intention to treat (ITT) population comprised 77 patients randomized to FOLFOX6 plus cetuximab and 74 randomized to FOLFIRI plus cetuximab. 1Values based on all treated patients (n = 151). FOLFOX: 5-fluorouracil (5-FU) folinic acid (FA) and oxaliplatin; FOLFIRI: 5-FU FA and irinotecan; PD: Progressive disease; AE: Adverse event.
- Citation: Ocvirk J, Brodowicz T, Wrba F, Ciuleanu TE, Kurteva G, Beslija S, Koza I, Pápai Z, Messinger D, Yilmaz U, Faluhelyi Z, Yalcin S, Papamichael D, Wenczl M, Mrsic-Krmpotic Z, Shacham-Shmueli E, Vrbanec D, Esser R, Scheithauer W, Zielinski CC. Cetuximab plus FOLFOX6 or FOLFIRI in metastatic colorectal cancer: CECOG trial. World J Gastroenterol 2010; 16(25): 3133-3143
- URL: https://www.wjgnet.com/1007-9327/full/v16/i25/3133.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i25.3133