Published online May 7, 2010. doi: 10.3748/wjg.v16.i17.2187
Revised: February 9, 2010
Accepted: February 16, 2010
Published online: May 7, 2010
Hepatocellular carcinoma (HCC) is an aggressive tumor with poor long-term prognosis. Here, we present an unusual patient with a solitary recurrence of HCC in the right kidney 12 years after the initial diagnosis. This illustrates the importance of considering late recurrence in patients with a history of HCC and the management of these metastases.
- Citation: Wong TCL, To KF, Hou SSM, Yip SKH, Ng CF. Late retroperitoneal recurrence of hepatocellular carcinoma 12 years after initial diagnosis. World J Gastroenterol 2010; 16(17): 2187-2189
- URL: https://www.wjgnet.com/1007-9327/full/v16/i17/2187.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i17.2187
Hepatocellular carcinoma (HCC) is an aggressive tumor with poor long-term prognosis. The reported 5-year recurrence rate ranges from 75% to 100%[1]. Therefore, extrahepatic metastasis of HCC is uncommon due to the highly malignant nature of the primary tumor. However, with advances in different treatment modalities for HCC, the incidence of extrahepatic metastasis appears to be increasing[2]. Nevertheless, most recurrences occur relatively early after the initial diagnosis and treatment. Here, we report a patient who presented with a renal tumor 12 years after the initial treatment of primary HCC, which turned out to be a solitary recurrence of the initial HCC. The management of these distant metastases is also highlighted.
A 61-year-old man who was a chronic hepatitis B carrier with Child’s A liver cirrhosis, first presented 12 years ago with hemoperitoneum secondary to ruptured HCC. Emergency laparotomy for hemostasis was performed. Non-anatomical resection of the liver tumor was subsequently performed 1 mo later after his condition was optimized. Pathology showed an 8 cm × 6 cm × 4 cm, moderately differentiated HCC with a clear resection margin. The postoperative serum α fetoprotein (AFP) level dropped from 1500 to 2 ng/mL.
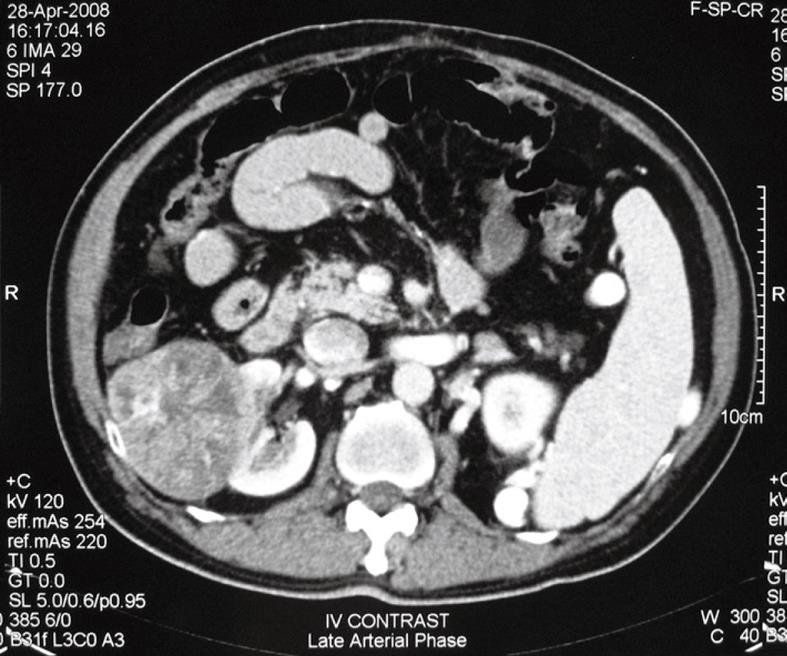
He was followed up regularly, 3-monthly for the first 2 years, followed by half-yearly follow-up. Serum AFP was measured during each follow-up and abdominal ultrasound was also performed during each follow-up for the first 5 years and then yearly during subsequent follow-up. There was no evidence of recurrence and the patient remained well clinically until 12 years later, when surveillance ultrasound revealed a right renal mass. Subsequent computed tomography showed a 5.3 cm pedunculated, well-circumscribed, contrast-enhanced soft tissue tumor in the right kidney, suspicious of renal cell carcinoma (RCC) (Figure 1). There was no space-occupying lesion in the liver or ascites. The tumor was asymptomatic and was not found on the last ultrasound performed 1 year ago. Serum AFP level was also increased from 3 (6 mo previously) to 27 ng/mL. However, in view of the long recurrence-free period and no evidence of local recurrence, the treatment plan was to closely monitor the raised AFP level and proceed to treatment of the renal mass.
With the past history of laparotomy and hepatectomy, retroperitoscopic nephrectomy was planned. During the operation, a large vascular, friable and exophytic tumor was found to have developed in the right kidney. However, the tumor was quite adhesive to the surrounding tissue, such that it inadvertently ruptured despite careful dissection. The surgery was then converted to an open procedure. The operation lasted 195 min with an estimated blood loss of 3 L. The post-operative course was uneventful and he was discharged 7 d after the operation.
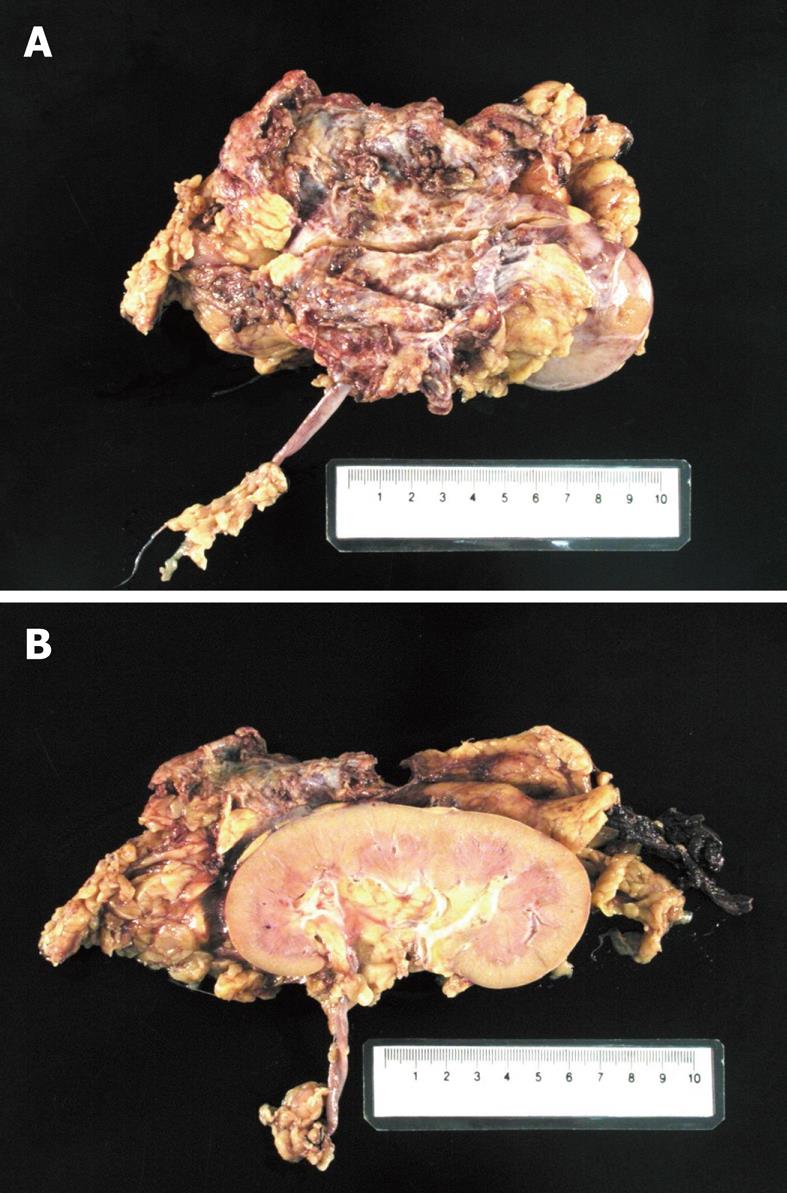
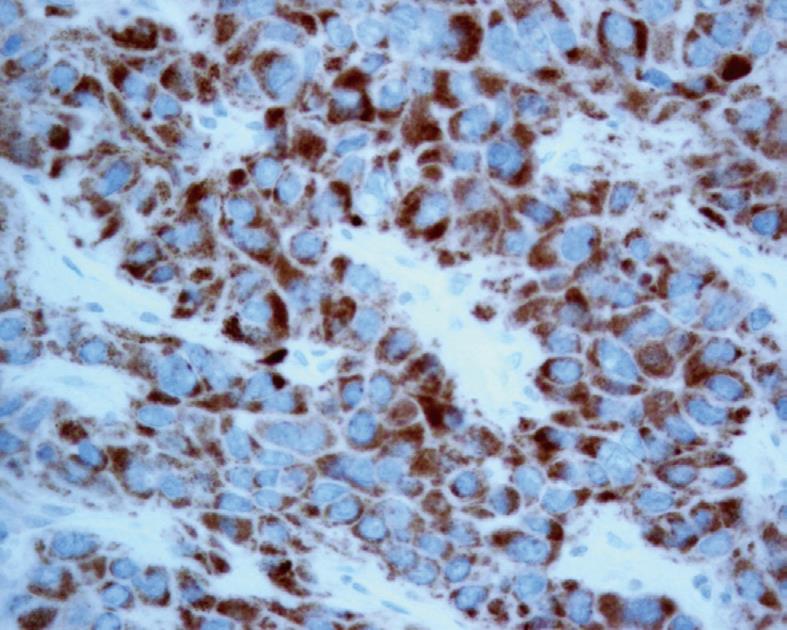
However, the subsequent unexpected pathology was found to be extra-hepatic metastasis of HCC at Gerota’s fascia. Macroscopically, the tumor was in the perinephric fat with no invasion to the renal capsule (Figure 2). Microscopic examination showed trabeculae of polygonal eosinophilic tumor cells, with epithelial cohesion, marked pleomorphism, associated with sinusoidal-type vascular channels and bile canaliculi were also identified. The kidney and pelvi-calyceal system were intact. When compared with the previously resected HCC 12 years ago, they both demonstrated similar microscopic characteristics and both were immunoreactive to polyclonal carcinoembryonic antigen and HepPar (Figure 3), but not to markers for RCC. Both the primary and recurrent HCC demonstrated the same TP53 mutation in exon 7 (R249S AGG > AGC). This result by itself was not confirmatory, but supported the clonal relationship of the primary and recurrent HCC in this case.
After nephrectomy, the patient’s serum AFP level dropped to 1 ng/mL, i.e. normal. As there was no evidence of other systemic recurrence, no adjuvant chemotherapy was administered. The patient remained well 18 mo after the nephrectomy and serum AFP remained normal. The latest positron-emission tomography performed at 1 year after nephrectomy showed no evidence of recurrence.
In the past, extrahepatic metastasis of HCC was considered a relatively uncommon phenomenon, owing to the highly malignant nature of the primary tumor. Nonetheless, with advances in treatment for HCC, its overall prognosis is improving and the incidence of extrahepatic metastasis appears to be increasing[3]. The commonest sites of extrahepatic metastases are lung, lymph node and bone followed by adrenal glands[4-6]. Extrahepatic metastasis can occur via three routes: hematogenous (56%), lymphogenous (27%) and direct invasion (22%) and more than one mode of spread can occur[7]. It is uncommon for HCC to spread to kidney and most believe that tumor spread to the kidneys is via the hematogenous route[8]. In our patient, the metastasis spread to the perinephric tissue without direct invasion of kidney parenchyma. It was postulated that the initial rupture of the primary liver tumor resulted in some seeding of tumor cells in the retroperitoneal region giving rise to subsequent tumor recurrence.
However, it is still extremely rare to have recurrent HCC 12 years after the initial diagnosis and treatment. The reported median duration between initial treatment for HCC to extrahepatic spread is 23.2 mo[1]. While there was a report suggesting that HCC can recur more than 6 years after initial management[9], there are no reports of recurrence occurring 10 years after initial treatment. It is difficult to explain the exceptionally long time lapse between treatment of the primary tumor and the current recurrence in our patient. It can not be explained even by shed cells belonging to a very low malignant clone of tumor cells.
Although there is no standard management protocol for patients with extrahepatic metastasis of HCC, it is generally believed that aggressive treatment for extrahepatic metastasis can prolong survival in selected patients with good performance status and limited volume of recurrence. In a review of patients with both intrahepatic and extrahepatic recurrent HCC, patients who received an aggressive combination of metastatectomy and locoregional therapy, with or without systemic therapy, had a better survival rate compared to those who received only non-surgical treatment (median survival after recurrence was 44.0 mo in the aggressive treatment group vs 10.6 mo in the non-surgically treated group)[2]. For patients with lung metastasis, it was also shown that a longer disease-free interval from primary treatment and a smaller number of metastasis (less than three) were associated with better prognosis[10]. In our patient, the solitary nature and long disease-free interval were relatively good prognostic factors.
Certainly, a more frequent follow-up protocol, especially imaging, may help to detect the lesion at an earlier stage. However, a careful balance between the yield and medical cost is needed, as late recurrence of HCC after a disease-free period of 5 years is still a very rare condition.
Our case illustrates that, in managing patients with a past history of HCC who present with new mass lesions, the diagnosis of extra-hepatic metastasis should always be suspected. Although the final treatment might not be altered, the diagnosis of metastatic HCC would allow better surgical planning and counseling for the patient.
Peer reviewers: Antonio Basoli, Professor, General Surgery “Paride Stefanini”, Università di Roma - Sapienza, Viale del Policlinico 155, Roma 00161, Italy; Giuseppe Currò, MD, University of Messina, Via Panoramica, 30/A, 98168 Messina, Italy
S- Editor Wang JL L- Editor Webster JR E- Editor Zheng XM
| 1. | Ishii H, Furuse J, Kinoshita T, Konishi M, Nakagohri T, Takahashi S, Gotohda N, Nakachi K, Yoshino M. Extrahepatic spread from hepatocellular carcinoma: who are candidates for aggressive anti-cancer treatment? Jpn J Clin Oncol. 2004;34:733-739. [Cited in This Article: ] |
| 2. | Poon RT, Fan ST, O'Suilleabhain CB, Wong J. Aggressive management of patients with extrahepatic and intrahepatic recurrences of hepatocellular carcinoma by combined resection and locoregional therapy. J Am Coll Surg. 2002;195:311-318. [Cited in This Article: ] |
| 3. | Kanda M, Tateishi R, Yoshida H, Sato T, Masuzaki R, Ohki T, Imamura J, Goto T, Yoshida H, Hamamura K. Extrahepatic metastasis of hepatocellular carcinoma: incidence and risk factors. Liver Int. 2008;28:1256-1263. [Cited in This Article: ] |
| 4. | Tanaka K, Shimada H, Matsuo K, Takeda K, Nagano Y, Togo S. Clinical features of hepatocellular carcinoma developing extrahepatic recurrences after curative resection. World J Surg. 2008;32:1738-1747. [Cited in This Article: ] |
| 5. | Uka K, Aikata H, Takaki S, Shirakawa H, Jeong SC, Yamashina K, Hiramatsu A, Kodama H, Takahashi S, Chayama K. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol. 2007;13:414-420. [Cited in This Article: ] |
| 6. | Natsuizaka M, Omura T, Akaike T, Kuwata Y, Yamazaki K, Sato T, Karino Y, Toyota J, Suga T, Asaka M. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol. 2005;20:1781-1787. [Cited in This Article: ] |
| 7. | Texler ML, Pierides J, Maddern GJ. Case report: A hepatocellular carcinoma metastasis in the distal pancreas. J Gastroenterol Hepatol. 1998;13:467-470. [Cited in This Article: ] |
| 8. | Lo CM, Lai EC, Fan ST, Choi TK, Wong J. Resection for extrahepatic recurrence of hepatocellular carcinoma. Br J Surg. 1994;81:1019-1021. [Cited in This Article: ] |
| 9. | Schreibman IR, Bejarano P, Martinez EJ, Regev A. Very late recurrence of hepatocellular carcinoma after liver transplantation: case report and literature review. Transplant Proc. 2006;38:3140-3143. [Cited in This Article: ] |
| 10. | Kuo SW, Chang YL, Huang PM, Hsu HH, Chen JS, Lee JM, Lee PH, Lee YC. Prognostic factors for pulmonary metastasectomy in hepatocellular carcinoma. Ann Surg Oncol. 2007;14:992-997. [Cited in This Article: ] |