Published online Apr 7, 2010. doi: 10.3748/wjg.v16.i13.1670
Revised: January 14, 2010
Accepted: January 21, 2010
Published online: April 7, 2010
We present the case of an 18-year-old female transferred to our center from an outside hospital due to persistent gastrointestinal bleeding. Two weeks prior to her transfer she underwent duodenal omentopexy for a perforated duodenal peptic ulcer. The patient underwent a computed tomography angiogram which identified the source of bleeding as a giant gastro-duodenal artery (GDA) pseudoaneurysm. The patient was taken to interventional radiology where successful microcoil embolization was performed. We present this rare case of a giant GDA pseudoaneurysm together with imaging and a review of the medical literature regarding prevalence, etiology and treatment options for visceral arterial aneurysms.
- Citation: Elazary R, Abu-Gazala M, Schlager A, Shussman N, Rivkind AI, Bloom AI. Therapeutic angiography for giant bleeding gastro-duodenal artery pseudoaneurysm. World J Gastroenterol 2010; 16(13): 1670-1672
- URL: https://www.wjgnet.com/1007-9327/full/v16/i13/1670.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i13.1670
Upper gastrointestinal bleeding (UGIB) is one of the leading causes of admission to the emergency department. While the vast majority of these bleeding episodes resolve spontaneously and most of the remaining cases are successfully managed endoscopically, a small percentage of patients require treatment using more invasive procedures such as therapeutic angiography and surgery. UGIB can be caused by a number of various pathologies. Gastro-duodenal artery (GDA) pseudoaneurysm is a rare but potentially fatal complication of surgery and, hence, early recognition and management of this complication is critical[1]. Although open surgery has traditionally been the treatment of choice, therapeutic angiography is emerging as an effective treatment modality for selected cases[2]. In this article we present such a case as well as remarkable images of the giant GDA aneurysm. Additionally, we discuss the pathophysiology of the condition, the treatment options and our rationale for opting for angiographic intervention over surgery.
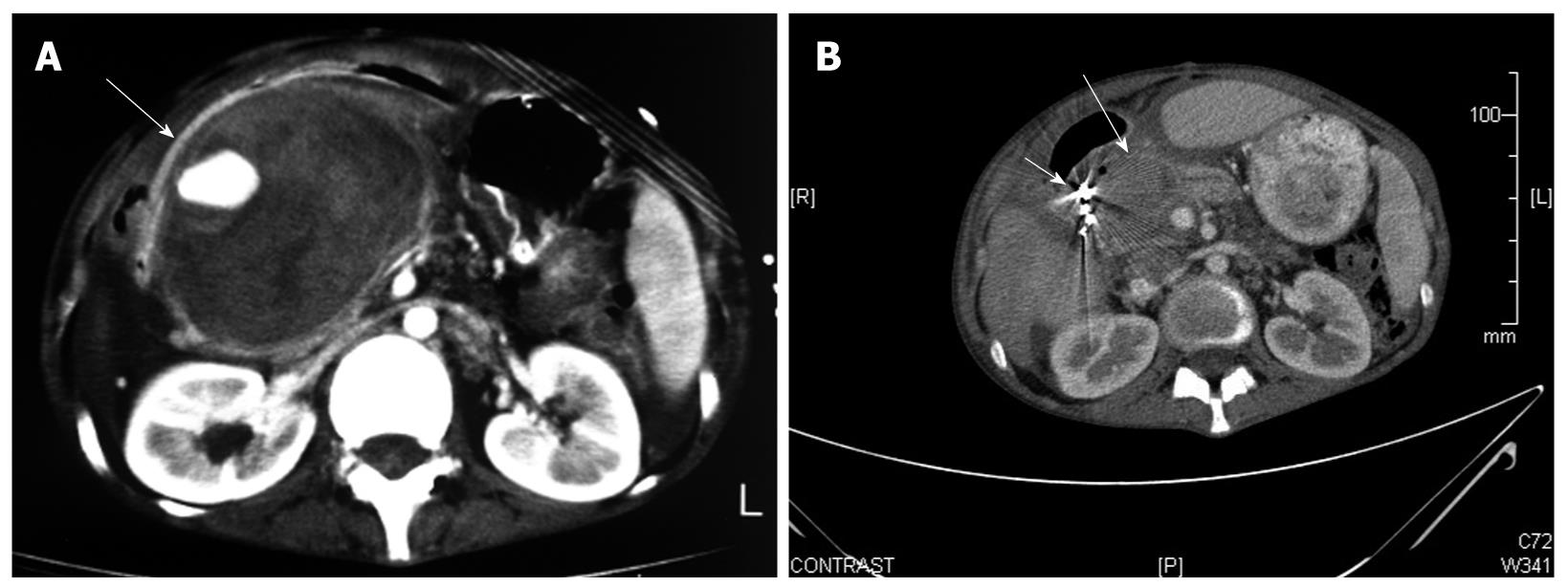
An 18-year-old female was transferred to our surgery department due to unsuccessful treatment of acute upper gastrointestinal bleeding. Seven months prior to this admission she was diagnosed with Crohn’s disease of the ileo-cecal region. She was treated with oral steroids and mesalamine (Pentasa) with gradual improvement of the disease symptoms. Five months later she was admitted to a local community hospital due to acute abdominal pain. She was taken immediately to the operating theatre. At that time, a perforated duodenal ulcer was identified and treated with duodenal omentopexy (Graham’s patch). On post-operative day 14, the patient began bleeding from the surgical wound and experiencing episodes of UGIB. She was transfused with multiple units of packed blood cells and was transferred to a second hospital where she underwent an upper endoscopy (Figure 1A). The endoscopy showed erosive gastritis and blood clots without active bleeding. Due to unsatisfactory explanation for the source of the hemorrhage she underwent computed tomography angiography (CTA) which demonstrated a giant pseudoaneurysm of the GDA, surrounded by fresh clots of blood. The patient was transfused with an additional five units of packed blood cells and transferred to our institution as the initial hospital did not have an interventional radiology unit. Upon arrival at the emergency department, the patient’s pulse was 80 bpm and blood pressure was 110/70 mmHg. A pulsatile abdominal mass was palpated at the right upper quadrant. Blood tests showed hemoglobin level of 11.6 mg/dL and normal coagulation studies. A naso-gastric tube was inserted which drained multiple clots of blood, at which time she was treated with intravenous omeprazole and taken to the angiography suite. After cannulation of the celiac trunk and the superior mesenteric artery via a right trans-femoral approach, the giant pseudoaneurysm emerging from the GDA was demonstrated. Arterial embolization was performed by placement of several detachable microcoils until flow-arrest was obtained. The procedure was performed without complication. Eight days following the procedure she underwent CTA which showed the angiographic coils surrounded by a small collection of fluid and no signs of perfusion in the GDA (Figure 1B). She was discharged on the 10th d of hospitalization. After two years of follow up, the patient is asymptomatic and has not been hospitalized due to recurrent UGIB.
Visceral artery pseudoaneurysms are considered rare pathologies. Nevertheless, pseudoaneurysm rupture and bleeding can lead to significant morbidity and mortality. The visceral arteries which most commonly develop pseudoaneurysm are the splenic artery, hepatic arteries, gastric and gastroepiploic arteries, gastro-duodenal artery and branches of the mesenteric arteries[3]. The most common artery involved is the splenic. GDA aneurysms are considered to be extremely rare. Although pseudoaneurysms and aneurysms of splanchnic arteries have traditionally been considered uncommon clinical entities, the prevalence discovered during autopsy study was surprisingly high (10.4%)[1]. More recent, higher rates of detection are likely related to increased frequency of imaging studies such as ultrasonography and computed tomography. There are multiple etiologies for development of pseudoaneurysms including: atherosclerosis, trauma, surgery, pancreatitis, infection, collagen vascular disease and congenital abnormalities. Infection and trauma are probably the most common causes. Infection can occur as a direct process around the vessel or as lymphatic spread from a primary focus such as Mycobacterium tuberculosis located away from the vessel[4]. Patients with pseudoaneurysm may be asymptomatic or present with symptoms of gastrointestinal bleeding, intraperitoneal hemorrhage, obstructive jaundice due to external pressure by the pseudoaneurysm, hematobilia and rupture into the portal vein.
A review of visceral aneurysms conducted by Moore et al[5] concluded that 35% of GDA aneurysms are ruptured at presentation, carrying a mortality rate of 21%. In the past, these pseudoaneurysms were treated surgically. However, in the last two decades, radiographic intervention has emerged as an attractive method for treating these patients. Surgical treatments include resection of the aneurysm and placing an interposition graft or performing aneurysmectomy with or without patching using a great saphenous vein. Endovascular treatments used are embolization or stent graft repair of the aneurysm. A laparoscopic procedure has also been reported for treating splenic artery aneurysm[6]. The potential complications of angiography, aside from those of standard femoral cannulation, include infarction of viscera and abscess formation. Fortunately, the complication rate regarding the former is relatively low due to rich collateral blood supply. Saltzberg et al[7] retrospectively reviewed the outcome of 65 patients diagnosed with visceral aneurysm, 18 of whom were treated by angiography and 9 by surgery. He reported that the initial technical success rate of the endovascular procedures was 94.4% (17/18). Major complications occurred in 22.2% (4/18). However, all four of these were in patients who were treated for splenic artery aneurysms. They concluded from their experience that endovascular management of visceral artery aneurysms is a reasonable alternative to open surgical repair, except for patients with splenic artery aneurysm. Carrafiello et al[8] published a case report of a patient with an evolving asymptomatic GDA pseudoaneurysm who underwent a combination of percutaneous, ultrasound-guided thrombin injection directly into the pseudoaneurysm sac and microcoil embolization of the gastro-duodenal artery. They concluded that thrombin injection may reduce the pseudoaneurysm’s wall tension and probably diminishes the risk for rupture.
Our patient presumably developed the pseudoaneurysm due to a leak occurring after the omentopexy repair for her perforated duodenal ulcer which initiated an infectious/inflammatory process in the region of the GDA area, causing damage to the vessel wall. Bleeding into the peritoneal space or the gastrointestinal tract beginning several days following an operation should be a cause for suspicion for the possibility of a ruptured pseudoaneurysm. Any operation which involves handling of an infected space or organ is a risk factor for infecting the peritoneal cavity and developing the pseudoaneurysm. Angioembolic occlusion of this giant aneurysm provided an effective treatment with minimal morbidity risk. Surgical repair in this case would have been particularly challenging and possibly morbid as it would have required re-exploring a previously operated space. We conclude that angiographic management is not only a feasible but possibly preferred treatment modality in selected cases of visceral artery aneurysms.
Peer reviewer: Klaus Thaler, MD, One Hospital Drive, McHany Hall, MC 413, Columbia, MO 65212, United States
S- Editor Tian L L- Editor Logan S E- Editor Ma WH
| 1. | Lee CH, Lan CC, Wang CC, Chan CY, Wu YK. Spontaneous rupture of gastroduodenal artery pseudoaneurysm following vigorous cough. Am J Gastroenterol. 2009;104:529-530. [Cited in This Article: ] |
| 2. | Salam TA, Lumsden AB, Martin LG, Smith RB 3rd. Nonoperative management of visceral aneurysms and pseudoaneurysms. Am J Surg. 1992;164:215-219. [Cited in This Article: ] |
| 3. | Chong WW, Tan SG, Htoo MM. Endovascular treatment of gastroduodenal artery aneurysm. Asian Cardiovasc Thorac Ann. 2008;16:68-72. [Cited in This Article: ] |
| 4. | Seith A, Gulati MS, Nandi B, Bhatia V, Garg PK, Bandhu S, Paul SB. Tuberculous pseudoaneurysm of gastroduodenal artery. Clin Imaging. 2003;27:408-410. [Cited in This Article: ] |
| 5. | Moore E, Matthews MR, Minion DJ, Quick R, Schwarcz TH, Loh FK, Endean ED. Surgical management of peripancreatic arterial aneurysms. J Vasc Surg. 2004;40:247-253. [Cited in This Article: ] |
| 6. | Pulli R, Dorigo W, Troisi N, Pratesi G, Innocenti AA, Pratesi C. Surgical treatment of visceral artery aneurysms: A 25-year experience. J Vasc Surg. 2008;48:334-342. [Cited in This Article: ] |
| 7. | Saltzberg SS, Maldonado TS, Lamparello PJ, Cayne NS, Nalbandian MM, Rosen RJ, Jacobowitz GR, Adelman MA, Gagne PJ, Riles TS. Is endovascular therapy the preferred treatment for all visceral artery aneurysms? Ann Vasc Surg. 2005;19:507-515. [Cited in This Article: ] |
| 8. | Carrafiello G, Laganà D, Recaldini C, Mangini M, Lumia D, Giorgianni A, Leonardi A, Fugazzola C. Combined percutaneous thrombin injection and endovascular treatment of gastroduodenal artery pseudoaneurysm (PAGD): case report. Emerg Radiol. 2007;14:51-54. [Cited in This Article: ] |