Published online Mar 7, 2009. doi: 10.3748/wjg.15.1085
Revised: January 17, 2009
Accepted: January 24, 2009
Published online: March 7, 2009
AIM: To assess the prevalence and location of advanced neoplasia in patients undergoing colonoscopy, and to compare the yield per indication.
METHODS: In a multicenter colonoscopy survey (n = 18 hospitals) in the Amsterdam area (Northern Holland), data of all colonoscopies performed during a three month period in 2005 were analyzed. The location and the histological features of all colonic neoplasia were recorded. The prevalence and the distribution of advanced colorectal neoplasia and differences in yield between indication clusters were evaluated. Advanced neoplasm was defined as adenoma > 10 mm in size, with > 25% villous features or with high-grade dysplasia or cancer.
RESULTS: A total of 4623 eligible patients underwent a total colonoscopy. The prevalence of advanced neoplasia was 13%, with 281 (6%) adenocarcinomas and 342 (7%) advanced adenomas. Sixty-seven percent and 33% of advanced neoplasia were located in the distal and proximal colon, respectively. Of all patients with right-sided advanced neoplasia (n = 228), 51% had a normal distal colon, whereas 27% had a synchronous distal adenoma. Ten percent of all colonoscopies were performed in asymptomatic patients, 7% of whom had advanced neoplasia. In the respective procedure indication clusters, the prevalence of right-sided advanced neoplasia ranged from 11%-57%.
CONCLUSION: One out of every 7-8 colonoscopies yielded an advanced colorectal neoplasm. Colonoscopy is warranted for the evaluation of both symptomatic and asymptomatic patients.
- Citation: Droste JSTS, Craanen ME, Hulst RWVD, Bartelsman JF, Bezemer DP, Cappendijk KR, Meijer GA, Morsink LM, Snel P, Tuynman HA, Wanrooy RLV, Wesdorp EI, Mulder CJ. Colonoscopic yield of colorectal neoplasia in daily clinical practice. World J Gastroenterol 2009; 15(9): 1085-1092
- URL: https://www.wjgnet.com/1007-9327/full/v15/i9/1085.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.1085
Colorectal cancer (CRC) is the second leading cause of cancer-related death in the Western world and the incidence in Asia is also rising[12]. In The Netherlands, 63 cases per 100 000 inhabitants were found in 2003, whereas the incidence in the United States was 52 cases per 100 000[34]. While several countries have already started nation-wide screening programs for colorectal cancer, in The Netherlands, the scale and mode of CRC screening are still being debated[5–8]. One issue is whether sigmoidoscopy or colonoscopy should be performed with particular emphasis on the potential differences in yield and spatial distribution of colorectal carcinomas and advanced adenomas. Before embarking on gradual implementation of any kind of endoscopic screening in The Netherlands, we need to understand the distribution of CRC as well as the high risk precursors within the colorectum. In recent advice to the government, the Health Council of The Netherlands acknowledged the importance of this issue, and indicated that additional research is required before commitment to a national screening program[6]. Although colonoscopy and sigmoidoscopy constitute a significant proportion of the endoscopic workload in daily clinical practice, the yield of pathology, except for highly selected populations, has not well been described[9]. In particular, international data are lacking real life incidence figures of both advanced and non-advanced colorectal neoplasia found in routine endoscopy programs. As a result, accurate data on CRC and its precursors obtained in this study could inform decisions in choosing a future screening modality and could facilitate future national research initiatives in the field of CRC.
Furthermore, in view of the relatively fixed endoscopic resources and the potential increase in endoscopic procedures related to a future CRC screening program, a clear insight into endoscopic utilization in daily clinical practice is mandatory. Colonoscopy is considered the gold standard for the evaluation of the symptomatic patient. However, indication clusters might predict the presence of advanced neoplasia located in the proximal or distal colon and, thus, might indicate whether a colonoscopy or a sigmoidoscopy is warranted. This insight might not only lead to changes in future manpower planning, but it might also lead to changes in endoscopic utilization depending on initial clinical indication, thereby potentially alleviating future endoscopic workload[10].
In the present study, we evaluated the diagnostic yield in terms of advanced and non-advanced neoplasia in a large cohort of Dutch patients referred for lower gastrointestinal (GI) endoscopy. Within this study, our primary objective was to assess the prevalence and location of advanced colorectal neoplasia in all patients clinically referred for colonoscopy. A secondary objective was to compare the yield of proximally located advanced neoplasia versus distally located advanced neoplasia in several indication clusters in total colonoscopies.
In this multicenter study, daily endoscopic clinical practice was prospectively monitored during a three month period in 2005 in the province Northern Holland (Amsterdam area). All colonoscopies and sigmoidoscopies performed in this time interval were evaluated. The province Northern Holland, serving a total community of 2 599 103 inhabitants (http://www.cbs.nl) has 18 hospitals (2 academic hospitals and 16 general/teaching hospitals). All 18 hospitals participated in this study. The study protocol was approved by the central medical ethics review board of the VU University Medical Centre in Amsterdam.
Age, gender, procedure indications, and endoscopic findings were obtained from all patients referred for lower GI endoscopy. All hospitals were visited every two weeks and all lower GI endoscopy reports between September 1st 2005 and December 1st 2005 were evaluated.
All examinations were performed by gastroenterologists, GI fellows, internists or colorectal surgeons. For the purpose of our analysis, the distal colon was defined as the rectum, sigmoid, and descending colon including the splenic flexure. The proximal colon was defined as the transverse colon, the ascending colon and the cecum, as assessed by the endoscopist. The percentage of complete colonoscopies was scored. Cecal intubation was considered a complete colonoscopy.
Indications for procedures were clustered in categories. In total, twelve clusters were defined as shown in Table 1. In many cases, more than one procedure indication was present. There was considerable overlap among the indications of abdominal pain (I), change in bowel habits (II), bloating (III), diarrhea (IV) and constipation (V). We defined an irritable bowel syndrome (IBS) cluster as including one or more of the above-mentioned symptoms (I-V), as has been described previously[7]. The IBS cluster excluded patients who underwent colonoscopy or sigmoidoscopy for surveillance of inflammatory bowel disease (IBD) or established IBD, weight loss, or GI bleeding [anemia/iron deficiency, positive fecal occult blood test (FOBT), hematochezia or melena].
| Indication cluster (n; %) | Consists of the following indications |
| G-I bleeding (696; 15) | Hematochezia and/or melena |
| Anemia (356; 8) | Any kind of anemia |
| CRC suspicion (204; 4) | Clinical and/or radiological suspicion CRC |
| Weight loss (101; 2) | Weight loss |
| Family history CRC1 (447; 10) | Any family history of CRC or screening |
| IBS (969; 21) | Abdominal pain, change in bowel habits, bloating, diarrhea, constipation |
| IBD exacerbation (256; 6) | Clinical suspicion IBD and/or endoscopic evaluation of IBD exacerbation |
| CRC surveillance (454; 10) | Follow up after CRC |
| Polyp surveillance (583; 13) | Follow up after polypectomy |
| IBD surveillance (142; 3) | Surveillance for dysplasia in IBD |
| FAP/HNPCC surveillance (84; 2) | Screening and/or surveillance in FAP/HNPCC families |
| Other/non-specified (331; 7) | No indication mentioned, ileus and desufflation therapy, fecal incontinence, monitoring diverticulitis after treatment, tenesmus, endoscopic treatment radiation enteritis |
All pathological and clinicopathological findings were categorized as indicating non-neoplastic mucosa (no polyps), hyperplastic polyps, adenomas with low-grade dysplasia, or advanced neoplasia. An advanced neoplasm was defined as an adenoma ≥ 1.0 cm, an adenoma with villous or tubulovillous architecture (≥ 25% villous component), an adenoma with high-grade dysplasia, or cancer. Advanced adenoma was defined as an adenoma ≥ 1.0 cm, an adenoma with villous or tubulovillous architecture (≥ 25% villous component) or an adenoma with high-grade dysplasia. Subsequently, the term advanced neoplasm was defined as comprising advanced adenomas and cancer, as has been described previously. A non-advanced neoplasm was defined as a hyperplastic polyp, an adenoma ≤ 1.0 cm with low-grade dysplasia or an adenoma ≤ 1.0 cm with ≤ 25% villous component of the architecture. Findings such as lipomas, lymphoid aggregates and inflammatory or juvenile polyps were categorized as indicating non-neoplastic mucosa. In the case of patients with more than one polyp in either the proximal or distal segment of the colon, the most advanced lesion in this particular segment was included in the analysis. The size of the polyp was estimated either with the use of open-biopsy forceps or on the basis of clinical judgement.
Pathology specimens were evaluated by local pathologists, who classified polyps according to the criteria established by the World Health Organization[11]. Pathology reports were accessible through the national pathology data system (PALGA)[12]. The prevalence and location of advanced neoplasia were assessed for all colonoscopies and for each indication cluster separately. All sigmoidoscopies and incomplete colonoscopies were excluded, except for incomplete colonoscopies due to an obstructing CRC. Other exclusion criteria were colonoscopies with insufficient bowel cleansing and colonoscopies in patients with a known advanced neoplasm in situ (procedure indication is polypectomy or endoscopic re-evaluation of the anatomic position of the tumor). In case a patient had undergone multiple colonoscopies, we only analyzed the examination in which the most advanced neoplastic lesion was found. In case a patient had a synchronous right-sided and left-sided advanced neoplasm, we only analyzed the most proximal lesion or we analyzed both synchronous lesions separately (Table 2).
| Indication cluster | Right-sided advanced neoplasia | Left-sided advanced neoplasia | Synchronous left- and right-sided advanced neoplasia | Total number of patients with advanced neoplasia |
| G-I bleeding (n = 696) | 19 (11) | 146 (83) | 11 (6) | 176 (25) |
| Anemia (n = 356) | 35 (57) | 21 (34) | 5 (8) | 61 (17) |
| CRC suspicion (n = 204) | 29 (33) | 54 (61) | 6 (7) | 89 (44) |
| Weight loss (n = 101) | 2 (22) | 7 (78) | 0 | 9 (9) |
| Family history CRC1 (n = 447) | 10 (30) | 19 (58) | 4 (12) | 33 (7) |
| IBS (n = 969) | 22 (26) | 57 (66) | 7 (8) | 86 (9) |
| IBD exacerbation (n = 256) | 1 (33) | 2 (67) | 0 | 3 (1) |
| CRC surveillance (n = 454) | 11 (29) | 23 (60) | 4 (11) | 38 (8) |
| Polyp surveillance (n = 583) | 29 (41) | 36 (51) | 6 (8) | 71 (12) |
| IBD surveillance (n = 142) | 3 (43) | 4 (57) | 0 | 7 (5) |
| FAP/HNPCC surveillance (n = 84) | 2 (25) | 5 (63) | 1 (13) | 8 (10) |
| Other/Non-specified2 (n = 331) | 20 (48) | 21 (50) | 1 (2) | 42 (13) |
| Total (n = 4623) | 183 (29) | 395 (63) | 45 (7) | 623 (13) |
Primary objective: In all successful total colonoscopies, the prevalence and location of advanced colorectal neoplasia and the age and gender of patients were assessed.
Secondary objective: For each indication cluster separately, the prevalence and location of advanced colorectal neoplasia were assessed in all successful total colonoscopies.
For comparison of proportions, the Fisher’s exact test or chi-square test with Yates correction were used. Analyses were performed with SPSS for Windows software, version 12.0 (SPSS Inc., Chicago, Illinois).
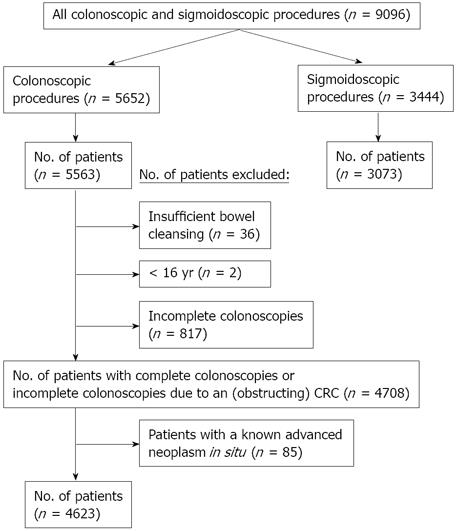
In total, 5652 colonoscopies and 3444 sigmoidoscopies were performed in 8636 patients during a three month period. Figure 1 shows an overview of the inclusion and exclusion criteria. After excluding all sigmoidoscopies (n = 3444), incomplete colonoscopies (n = 817), patients with insufficient bowel cleansing (n = 36), patients < 16 years (n = 2) and patients with a known advanced neoplasm in situ [procedure indication is polypectomy or endoscopic re-evaluation of anatomic position of the tumor (n = 85)], the total study cohort consisted of 4623 patients (mean age ± SD 58.8 ± 16 years, range 16-100 years). In 4 patients (0.1%), the age was not mentioned in the endoscopy report. Forty-seven percent and 53% of the patients were male and female, respectively (mean age for males 59.3 ± 15 years, mean age for females 58.4 ± 16 years, P = NS). In 0.1% of the patients (n = 4) gender was not mentioned in the endoscopy report. In 16% and 84% of the patients, endoscopies were performed in an academic hospital and general/teaching hospital, respectively. In all patients undergoing colonoscopy, the cecal intubation rate was 83%. In 14% of the cases, the cecum was not visualized and in 3% of the cases the issue was not accounted for in the colonoscopy report.
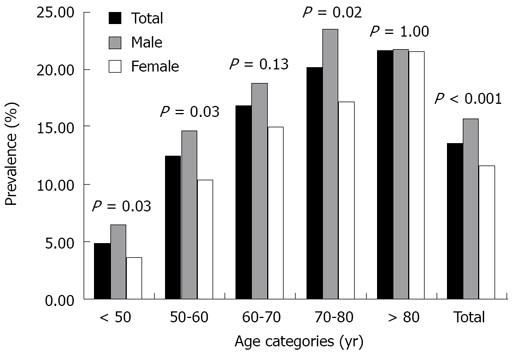
The prevalence and distribution of CRCs, advanced adenomas and advanced neoplasia are listed in Table 3. Furthermore, in all complete colonoscopies, three incident cases of carcinoid tumors, one anal carcinoma and three metastatic lesions of other primary tumors were detected. In patients with CRC (n = 281), 52% were males (mean age ± SD 68.0 ± 11 years) and 48% were females (mean age ± SD 70.6 ± 12 years) (P = NS). In patients with CRC, the tumor was located in the distal colon and proximal colon in 65% and 35% of cases, respectively. Of all patients with right-sided advanced neoplasia (i.e. advanced adenomas and/or cancer, n = 228), 51% had a normal appearing distal colon, whereas 49% had a synchronous distal polyp (41% advanced neoplasm, 14% small adenoma, 12% hyperplastic polyp and 33% non-specified polyp). Overall, 2.5% of the total study cohort had a proximally located advanced neoplasm without a synchronous distal polyp. Figure 2 illustrates the prevalence of advanced neoplasia in different age categories for males and females. Advanced neoplasia became more prevalent with increasing age. In 22% of the patients over 80 years an advanced neoplasm was found, compared to 5% of the patients under 50 years (P < 0.0001) Men were more likely than women to have advanced neoplasia (15.6% for men versus 11.6% for women, odds ratio (OR) corrected for age is 1.4; 95% confidence interval (CI) 1.18 to 1.67; P < 0.0001).
| Localization in the colo-rectum | CRC | Advanced adenoma | Advanced neoplasia1 |
| Rectum | 86 (31) | 74 (22) | 160 (26) |
| Sigmoid | 77 (27) | 131 (38) | 208 (33) |
| Descending colon | 19 (7) | 29 (8) | 48 (8) |
| Transverse colon | 23 (8) | 19 (6) | 42 (7) |
| Ascending colon | 37 (13) | 46 (13) | 83 (13) |
| Caecum | 39 (14) | 43 (13) | 82 (13) |
| Total | 281 (100) | 342 (100) | 623 (100) |
The yield and distribution of advanced neoplasia are summarized per indication cluster in Table 2. Advanced neoplasia were found in 25% of patients who presented with GI bleeding. Moreover, in cases of a clinical or radiological suspicion of CRC, the yield of advanced neoplasia was 44%. In all of the procedure indication clusters, the prevalence of right-sided advanced neoplasia ranged from 11%-57%. In patients who presented with GI bleeding, predominantly left sided advanced neoplasia were found (83%). In contrast, in patients who presented with anemia mostly right-sided advanced neoplasias were encountered (57%, P < 0.001). Advanced neoplasia were found in 7% of asymptomatic patients (10% of the total study cohort), who presented with a family history of CRC or with a CRC screening request. Finally, both left- and right-sided advanced neoplasias were found in 7% of all patients.
This study included all procedures from patients clinically referred for colonoscopy in a three month period. In The Netherlands, all colonoscopies are performed in a hospital setting (academic, teaching or general hospitals). No other institutions, like private practices or doctor’s offices, perform endoscopies. Our study includes all colonoscopies performed in Northern Holland, representing a large unselected sample of the population of The Netherlands. Therefore, our data accurately represent the entire lower GI endoscopic practice in Northern Holland which we regard to be representative for the whole of The Netherlands. This study cohort yielded 281 CRCs. However, all CRCs found using sigmoidoscopy (n = 95) or during abdominal surgery without prior endoscopy (n = 38), were excluded (data not shown in results section). Extrapolation of the total number of CRCs found to annual incidence figures would show a substantial increase in incidence of CRC compared to national/regional cancer registries (67 cases per 100 000 inhabitants compared to 63 cases per 100 000 inhabitants in 2003)[3]. In line with international data on the rising incidence of CRC, this finding would emphasize the importance of implementing a CRC screening program in The Netherlands to improve survival by diagnosing CRC or its precursors at an earlier stage[413].
In this referral population, more than 13% of colonoscopies performed yielded an advanced neoplasm (CRC/advanced adenoma). Of all advanced neoplasia found, 33% were located in the proximal colon and 67% were located in the distal colon. Similar to other studies, we identified male sex and increasing age as independent risk factors of advanced neoplasia, either distally located or proximally located[14–17]. However, male sex adjusted for age and distal findings did not significantly increase the risk of advanced proximal neoplasia. As shown in Figure 2, at least a 4-fold increase in prevalence of advanced neoplasia was observed in patients > 70 years compared with those of < 50 years. This age-related increase in prevalence of advanced neoplasia is in keeping with previous Western and Asian reports[18–20]. Unfortunately, the presence of a right-sided advanced neoplasm can not be adequately predicted by distal colonoscopic findings since 51% of proximally located advanced neoplasia had no distal polyps. If distal adenomas are considered sentinel lesions that warrant a complete colonoscopy, the percentage of detected proximally located advanced neoplasms would have been 27% if only sigmoidoscopy had been performed. However, in this study, a substantial proportion of distal polyps was not specified, which could be an important confounder (33% of all distal polyps in patients with proximally located advanced neoplasia). In accordance with other studies in which patients were referred for colonoscopy, no significant differences in the prevalence of proximally located advanced neoplasia with a normal appearing distal colon were found [prevalence of isolated, proximal advanced neoplasia in the United States (2.7%), Asia (2.2%) and The Netherlands (2.5%)][1721]. Moreover, compared to the Asian situation, no significantly different percentages were observed in terms of missed proximally located advanced neoplasia if only sigmoidoscopy had been carried out[17].
When taking into account the different procedure indication clusters, the prevalence of proximally located advanced neoplasia ranged from 11%-57%. In patients who presented with GI bleeding, only 11% of advanced neoplasia were located in the proximal colon, while 83% were located in the distal colon (P < 0.001). Six percent of the patients with GI bleeding had synchronous advanced neoplasia in both the distal and the proximal colon. Consequently, the majority of advanced neoplasia in patients with GI bleeding are found within reach of the sigmoidoscope. However, apart from GI bleeding, which is one of the most frequent procedure indications, right-sided advanced neoplasia is a common finding which cannot be ignored when considering the proper endoscopic procedure in clinical practice. Even in the IBS indication cluster, which has a low pretest likelihood ratio for advanced neoplasia[2223], similar percentages of right-sided advanced neoplasia were found compared to indications such as weight loss, family history of CRC, CRC surveillance and FAP/HNPCC surveillance (P = N.S.). A change in bowel habits was included in the IBS indication cluster (Table 1) which may be responsible for the high yield of advanced neoplasia, particularly in patients > 50 years. Surprisingly, there were hardly any referrals for colonoscopy based on a positive FOBT result (n < 10), which is a frequent procedure indication in other studies[7]. In all probability, this is due to a lack of confidence in the FOBT as a diagnostic test in The Netherlands. We hypothesize that this finding might also reflect the Dutch lagging behind in CRC awareness and pre-screening activities compared to other European countries[2425].
In this accurate regional representation of Dutch endoscopic practice, 10% of all colonoscopies in routine endoscopy programs were performed in asymptomatic patients. This ranged from an individual screening request without family history of CRC to a request because of a history of CRC in a 1st-3rd degree family relative. The diagnostic yield in terms of advanced neoplasia in this indication cluster was substantial (7%), and right-sided advanced neoplasms were frequently found (30%). Taking into account the yield of right-sided advanced neoplasia in each indication cluster, and in asymptomatic patients in particular, it can be argued whether these findings would be truly different in a CRC screening setting. To further elaborate on this conclusion, the majority of advanced colorectal adenomas and a proportion of early cancers are asymptomatic. These neoplasias are detected by chance during colonoscopy. Therefore, the topographic distribution and epidemiology of colorectal neoplasia, particularly advanced adenomas, found in this study should largely reflect the actual situation in The Netherlands where screening colonoscopy is non existent. This also means that this study could not have been performed in a screening population only. However, because of the increasing attention to CRC screening, in both policy makers, medical doctors and the general population, a substantial number of endoscopies are performed in daily clinical practice in asymptomatic patients. To a certain extent, our asymptomatic patients are comparable to a screening population. Therefore, this study contains an informative mix of symptomatic and asymptomatic patients with a comparable distribution rate of advanced neoplasia.
Our findings should be interpreted taking into account several potential caveats in case of extrapolation to a screening setting. Firstly, in this study the majority of patients were symptomatic or in a surveillance program which may be accompanied by a higher likelihood of having colorectal neoplasia. In contrast to screening colonoscopy, in which age limits are restricted, the wide age range of our study population may have influenced the rate of advanced colorectal neoplasia. The Dutch Health Council, however, asked for such routine endoscopy data before implementing a CRC screening program. Secondly, histology reports were generated by local pathologists meaning that there was an inherent risk of inter-observer variability in characterization of the histological types and degrees of dysplasia of polyps[2627]. Furthermore, in the total study cohort 331 patients had a non-specified polyp (7% of all patients and 18% of all colorectal neoplasms). Non-specification was mainly due to insufficient retrieval of snared polyps, lack of biopsies and poor quality of biopsy specimens. Although the percentage of advanced neoplasia that are missed because of non-specification remains elusive, the high number of non-specified polyps is rather worrisome for routine practice. Thirdly, polyp size is frequently misjudged by endoscopists[28]. In our study, no systematic size estimate was used and, therefore, an arbitrary cut-off value of 10 mm was used for discrimination of small and large polyps, leaving judgement of sizes to each endoscopist individually. Thus, due to the lack of predefined standardization, the proportion of truly advanced neoplasia may not be accurately reflected in this cohort.
Surprisingly, colonoscopy in daily clinical practice was incomplete in 17% of cases. Whether an incomplete colonoscopy was followed by a double-contrast barium enema or CT colonography to visualize the total colon is not known. Major contributors to cecal intubation failure were inflammation due to IBD or diverticulitis, extensive diverticular disease, stenosis/adhesions after abdominal surgery and large advanced adenomas. Undoubtedly, the miss rate of advanced neoplasia due to incomplete colonoscopies needs further clarification. Such low cecal intubation rates may frustrate future colonoscopy-based screening programs. Recently, simple measures have been proposed to optimise quality in colonoscopy[2930]. These studies and this low cecal intubation rate underscore the importance of continuous quality control in terms of reporting and appropriate training.
In conclusion, this study is an exact representation of daily clinical practice, and as such provides relevant data on the performance of colonoscopy with respect to the detection of advanced neoplasia. Our data are mandatory for the future planning of CRC screening in The Netherlands. Although this referral population may have a higher pre-test likelihood for colorectal neoplasia, the distribution of these lesions throughout the colorectum may be the same. At present, 10% of all colonoscopies in routine endoscopy programs are performed in asymptomatic patients with a substantial yield of advanced neoplasia. Based on clinical indication, no significant changes in endoscopic utilization can be realized to alleviate endoscopic workload since substantial numbers of right-sided advanced neoplasia are found in each indication cluster. Extrapolation of our data indicates that sigmoidoscopy would miss 33% of advanced neoplasia. Hence, our data show that colonoscopy is warranted for the evaluation of both symptomatic and asymptomatic patients.
Colorectal cancer (CRC) awareness accounts for an increasing number of colonoscopies performed in asymptomatic patients with a screening request or family history of CRC in The Netherlands. Before embarking on endoscopic screening, we need to understand the distribution of CRC as well as the high risk precursor lesions within the colorectum. International data are scarce regarding real life incidence figures of colorectal neoplasia found in routine endoscopy programs, evaluating both symptomatic and asymptomatic patients.
Knowledge of the incidence and distribution of CRC and high-risk precursor lesions in the colo-rectum in both symptomatic and asymptomatic patients, could tailor endoscopic utilization. Furthermore, it could facilitate making informed decisions in choosing a future screening modality and future national research initiatives in the field of CRC.
The overall yield in advanced neoplasia was significantly higher in this study than in the Asian situation (13.5% vs 9.4%). In accordance with Unite States and Asian studies, in which patients were referred for colonoscopy, high percentages of proximally located advanced neoplasia with a normal appearing distal colon were found. Extrapolation of our data indicates that sigmoidoscopy would miss 33% of advanced neoplasia. The yield of advanced neoplasia in asymptomatic patients is substantial (7%). Although this referral population may have a higher pre-test likelihood of colorectal neoplasia compared to a screening population, the distribution of these lesions throughout the colorectum may be the same. In The Netherlands, where screening colonoscopy is non existent, 10% of all colonoscopies in routine endoscopy programs are performed in asymptomatic patients.
This study shows that colonoscopy has a high yield in detecting advanced colorectal neoplasia in daily clinical practice. Colonoscopy is warranted for the evaluation of both symptomatic and asymptomatic patients, since substantial numbers of right-sided advanced neoplasia are found in both patient groups. These data are mandatory for the future planning of CRC screening in The Netherlands.
Advanced colorectal adenoma is defined as an adenoma ≥ 1.0 cm, an adenoma with villous or tubulovillous architecture (≥ 25% villous component) or an adenoma with high-grade dysplasia. Advanced colorectal neoplasm is defined as an adenoma ≥ 1.0 cm, an adenoma with tubulovillous or villous architecture (≥ 25% villous component), an adenoma with high-grade dysplasia, or cancer. Subsequently, the term advanced colorectal neoplasm was defined as comprising advanced adenomas and cancer.
This is a useful study reporting the prevalence of colonic lesions in symptomatic and asymptomatic patients in a Dutch province containing 18 hospitals. The sample size is large, and it is fairly well written.
| 1. | Sung JJ, Lau JY, Goh KL, Leung WK. Increasing incidence of colorectal cancer in Asia: implications for screening. Lancet Oncol. 2005;6:871-876. [Cited in This Article: ] |
| 2. | Yiu HY, Whittemore AS, Shibata A. Increasing colorectal cancer incidence rates in Japan. Int J Cancer. 2004;109:777-781. [Cited in This Article: ] |
| 3. | Siesling S, van der Aa MA, Coebergh JW, Pukkala E. Time-space trends in cancer incidence in the Netherlands in 1989-2003. Int J Cancer. 2008;122:2106-2114. [Cited in This Article: ] |
| 4. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96. [Cited in This Article: ] |
| 5. | Malila N, Anttila A, Hakama M. Colorectal cancer screening in Finland: details of the national screening programme implemented in Autumn 2004. J Med Screen. 2005;12:28-32. [Cited in This Article: ] |
| 6. | de Visser M, van Ballegooijen M, Bloemers SM, van Deventer SJ, Jansen JB, Jespersen J, Kluft C, Meijer GA, Stoker J, de Valk GA. Report on the Dutch consensus development meeting for implementation and further development of population screening for colorectal cancer based on FOBT. Cell Oncol. 2005;27:17-29. [Cited in This Article: ] |
| 7. | Lieberman DA, Holub J, Eisen G, Kraemer D, Morris CD. Utilization of colonoscopy in the United States: results from a national consortium. Gastrointest Endosc. 2005;62:875-883. [Cited in This Article: ] |
| 8. | Bretthauer M, Gondal G, Larsen K, Carlsen E, Eide TJ, Grotmol T, Skovlund E, Tveit KM, Vatn MH, Hoff G. Design, organization and management of a controlled population screening study for detection of colorectal neoplasia: attendance rates in the NORCCAP study (Norwegian Colo-rectal Cancer Prevention). Scand J Gastroenterol. 2002;37:568-573. [Cited in This Article: ] |
| 9. | Seow CH, Ee HC, Willson AB, Yusoff IF. Repeat colonoscopy has a low yield even in symptomatic patients. Gastrointest Endosc. 2006;64:941-947. [Cited in This Article: ] |
| 10. | Mysliwiec PA, Brown ML, Klabunde CN, Ransohoff DF. Are physicians doing too much colonoscopy? A national survey of colorectal surveillance after polypectomy. Ann Intern Med. 2004;141:264-271. [Cited in This Article: ] |
| 11. | Konishi F, Morson BC. Pathology of colorectal adenomas: a colonoscopic survey. J Clin Pathol. 1982;35:830-841. [Cited in This Article: ] |
| 12. | Casparie M, Tiebosch AT, Burger G, Blauwgeers H, van de Pol A, van Krieken JH, Meijer GA. Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol. 2007;29:19-24. [Cited in This Article: ] |
| 13. | Lin OS, Kozarek RA, Schembre DB, Ayub K, Gluck M, Drennan F, Soon MS, Rabeneck L. Screening colonoscopy in very elderly patients: prevalence of neoplasia and estimated impact on life expectancy. JAMA. 2006;295:2357-2365. [Cited in This Article: ] |
| 14. | Regula J, Rupinski M, Kraszewska E, Polkowski M, Pachlewski J, Orlowska J, Nowacki MP, Butruk E. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med. 2006;355:1863-1872. [Cited in This Article: ] |
| 15. | Imperiale TF, Wagner DR, Lin CY, Larkin GN, Rogge JD, Ransohoff DF. Using risk for advanced proximal colonic neoplasia to tailor endoscopic screening for colorectal cancer. Ann Intern Med. 2003;139:959-965. [Cited in This Article: ] |
| 16. | Atkin WS, Morson BC, Cuzick J. Long-term risk of colorectal cancer after excision of rectosigmoid adenomas. N Engl J Med. 1992;326:658-662. [Cited in This Article: ] |
| 17. | Leung WK, Ho KY, Kim WH, Lau JY, Ong E, Hilmi I, Kullavanijaya P, Wang CY, Li CJ, Fujita R. Colorectal neoplasia in Asia: a multicenter colonoscopy survey in symptomatic patients. Gastrointest Endosc. 2006;64:751-759. [Cited in This Article: ] |
| 18. | Lieberman DA, Prindiville S, Weiss DG, Willett W. Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA. 2003;290:2959-2967. [Cited in This Article: ] |
| 19. | Sung JJ, Chan FK, Leung WK, Wu JC, Lau JY, Ching J, To KF, Lee YT, Luk YW, Kung NN. Screening for colorectal cancer in Chinese: comparison of fecal occult blood test, flexible sigmoidoscopy, and colonoscopy. Gastroenterology. 2003;124:608-614. [Cited in This Article: ] |
| 20. | Chiu HM, Wang HP, Lee YC, Huang SP, Lai YP, Shun CT, Chen MF, Wu MS, Lin JT. A prospective study of the frequency and the topographical distribution of colon neoplasia in asymptomatic average-risk Chinese adults as determined by colonoscopic screening. Gastrointest Endosc. 2005;61:547-553. [Cited in This Article: ] |
| 21. | Anderson JC, Alpern Z, Messina CR, Lane B, Hubbard P, Grimson R, Ells PF, Brand DL. Predictors of proximal neoplasia in patients without distal adenomatous pathology. Am J Gastroenterol. 2004;99:472-477. [Cited in This Article: ] |
| 22. | Rex DK. Colonoscopy: a review of its yield for cancers and adenomas by indication. Am J Gastroenterol. 1995;90:353-365. [Cited in This Article: ] |
| 23. | Lieberman DA, de Garmo PL, Fleischer DE, Eisen GM, Chan BK, Helfand M. Colonic neoplasia in patients with nonspecific GI symptoms. Gastrointest Endosc. 2000;51:647-651. [Cited in This Article: ] |
| 24. | Keighley MR, O’Morain C, Giacosa A, Ashorn M, Burroughs A, Crespi M, Delvaux M, Faivre J, Hagenmuller F, Lamy V. Public awareness of risk factors and screening for colorectal cancer in Europe. Eur J Cancer Prev. 2004;13:257-262. [Cited in This Article: ] |
| 25. | Coebergh JW. Colorectal cancer screening in Europe: first things first. Eur J Cancer. 2004;40:638-642. [Cited in This Article: ] |
| 26. | Yoon H, Martin A, Benamouzig R, Longchampt E, Deyra J, Chaussade S. [Inter-observer agreement on histological diagnosis of colorectal polyps: the APACC study]. Gastroenterol Clin Biol. 2002;26:220-224. [Cited in This Article: ] |
| 27. | Terry MB, Neugut AI, Bostick RM, Potter JD, Haile RW, Fenoglio-Preiser CM. Reliability in the classification of advanced colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 2002;11:660-663. [Cited in This Article: ] |
| 28. | Schoen RE, Gerber LD, Margulies C. The pathologic measurement of polyp size is preferable to the endoscopic estimate. Gastrointest Endosc. 1997;46:492-496. [Cited in This Article: ] |
| 29. | West NJ, Poullis AP, Leicester RJ. The NHS Bowel Cancer Screening Programme--a realistic approach with additional benefits. Colorectal Dis. 2008;10:708-714. [Cited in This Article: ] |
| 30. | Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006;355:2533-2541. [Cited in This Article: ] |