INTRODUCTION
Cystic neoplasms of the pancreas are relatively rare, comprising 10 percent of pancreatic cysts and only 1 percent of pancreatic cancers. Cystic neoplasms encompasses mucinous cystic neoplasms, serous cystadenomas, papillary cystic tumors, cystic islet cell tumors and intraductal papillary mucinous neoplasms of the pancreas (IPMNs)[12].
IPMN was first described in Japan in 1982, when four patients, diagnosed with pancreatic carcinoma with favorable outcomes, were reported sharing three distinct clinical features: dilated main pancreatic duct, patulous ampullary orifice and mucus secretion[3]. Since then, several reports have described mucus-producing pancreatic tumors with different stages of carcinogenesis, referred to by a variety of names[4–10], but mostly as mucinous ductal cancers. IPMN has very clear malignant potential and exhibits a broad histological spectrum, ranging from minimal mucinous hyperplasia to adenoma to invasive carcinoma[11–13].
The criteria used to classify IPMNs and to discriminate them from other mucin-producing cystic neoplasms of the pancreas have been established by the WHO in 1996[14]. The WHO gave a clear definition for IPMNs as intraductal mucin-producing neoplasms with tall, columnar, mucin-containing epithelium, with or without papillary projections. This type of neoplasm involves the main pancreatic duct and/or major side branches.
The majority of IPMNs are located in the pancreatic head (75%), while the rest involves the body/tail regions[15]. Multifocal neoplasms have been hypothesized[15], but the real prevalence of multifocality is unknown. Here, we present a 72-year-old male diagnosed with non-invasive mixed type IPMN (carcinoma in situ) in the pancreatic head together with a branch duct type IPMN (duct atypia) in the pancreatic body and tail.
CASE REPORT
A 72 years old male presented in 2005 with abdominal fullness after meals, general weakness, and body weight loss of about 2-3 kg occurred during the previous 3 mo. He had had diabetes and hypertension under regular medical control for the past 8 years. He was first admitted as outpatient, and an upper panendoscopy was performed, which showed chronic duodenal ulcer and gastric erosions. Antacids were prescribed but symptoms were not relieved.
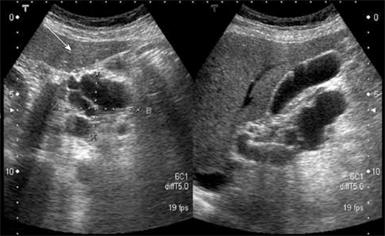
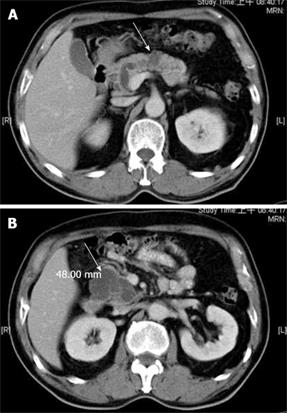
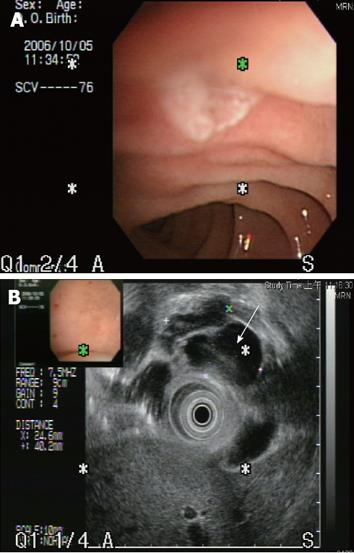
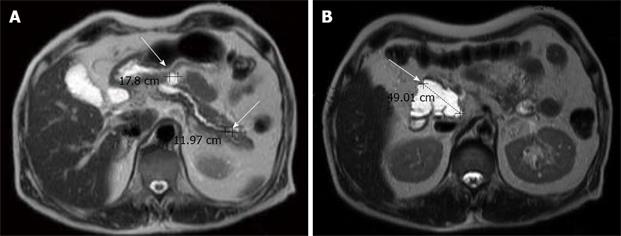
Abdominal ultrosonography was then carried out (Figure 1) and a pancreatic head cystic tumor with pancreatic duct dilatation (0.75 cm) was found. He was then hospitalized for further treatment. The physical examination revealed a soft abdomen without palpable mass. No lymphadenopathy was found over his neck or the axillary and inguinal areas. Digital examination showed nothing abnormal. The following laboratory tests were performed: white blood cell count 9000/mm3, hemoglobin 13.5 g/dL, platelet count 257 000/mm3, albumin 4.1 g/dL, aspartate aminotrasferase (AST) 16 U/L, alanine aminotransferase (ALT) 14 U/L, alkaline phosphatase 47 U/L, total bilirubin 0.6 mg/dL, creatinine 0.7 mg/dL, amylase 117 U/L, lipase 48 U/L. Tumor markers, including carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA 19-9), were also checked and their results were 0.92 ng/mL (normal < 5 ng/mL) and 27.2 U/mL (normal < 37 U/mL), respectively. Then abdominal computed tomography (CT) was performed (Figure 2), which showed two cystic tumors at the level of the pancreatic head and body, measuring 4.8 cm and 1.2 cm, respectively. To further investigate the nature of the tumors, endoscope ultrosonography (EUS) and magnetic resonance imaging (MRI) were carried out. The EUS (Figure 3) disclosed a cystic tumor, 4 cm × 4.2 cm, without obvious internal projective mass in the pancreatic head. The papilla of Vater was grossly normal without mucin discharge. The MRI (Figure 4) showed three cystic lesions in pancreatic head (4.9 cm), body (1.8 cm) and tail (1.2 cm) with pancreatic duct dilatation. Given the presence of pancreas cystic neoplasms, which may be malignant IPMNs, cystic adenocarcinomas or cystic adenomas, affecting the pancreatic head, body and tail, an extended Whipple operation (including the partial resection of the pancreatic body, about 2 cm away from the portal vein) together with a distal pancreatectomy were performed. Intraoperative findings revealed three tumors as described in the MRI. Intraoperative pancreatic US revealed no additional cystic mass in the residual pancreas, measuring about 7 cm in length, after resection. The proximal and distal margins were all negative for malignancy demonstrated by intraoperative histopathologic examination. The regional lymph nodes were grossly normal.
Figure 1 Abdominal ultrosonograpy showing multiple cystic tumors over the pancreatic head area with P-duct dilatation.
Figure 2 Abdominal computed tomography showing pancreatic head and body cystic tumors (arrow) with no evidence of regional lymphadenopathy or distant metastases.
Figure 3 The endoscopic ultrasonogaphy showed that the papilla of Vater was grossly normal without mucin discharge.
However, a cystic tumor (arrow) measuring 4.0 cm x 4.2 cm in size was seen over the pancreatic head. The lining of cysts showed no definite projectile mass and the pancreatic duct was dilated about 0.6 cm in the head and 0.4 cm in the body and tail area.
Figure 4 Magnetic resonance imaging showing three cystic lesions in the pancreatic head (4.
9 cm), body (1.8 cm) and tail (1.2 cm), with P-duct dilatation. Mild common bile duct and common hepatic duct dilatation without definite tumor was observed in the liver upon MRI.
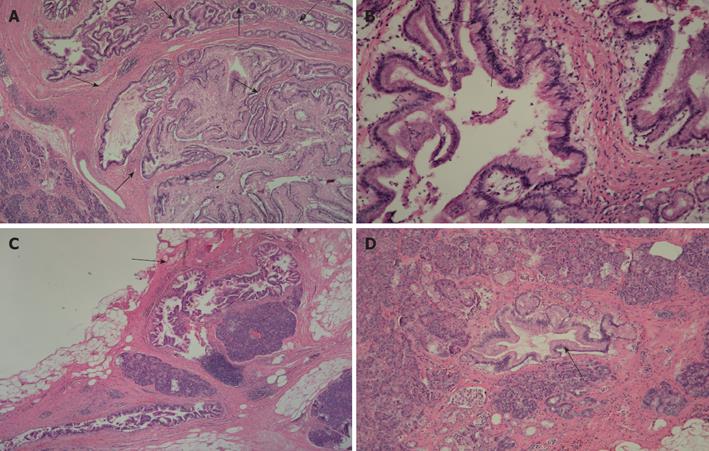
Histopathologic examination revealed that the pancreatic head showed the presence of cystically dilated ducts, encompassing the main and branch ducts, with formation of papillary growth lined by mucin-secreting columnar epithelium. The mucin-secreting epithelium had stratification of atypical nuclei. No obvious stromal invasion was seen. The features suggested a diagnosis of intraductal papillary mucinous carcinoma, non-invasive type (Figure 5A, B). The pancreatic body was focally involved by the lesion (Figure 5C), in the branch duct, not contiguous to the lesion of the pancreatic head. The pancreatic tail displayed focal presence of atypical ducts (Figure 5D), located in the branch duct. The final pathological diagnosis was non invasive mixed (involving both the main and the branch duct) type IPMN (carcinoma in situ) in the pancreatic head and branch duct type IPMN (duct atypia) in the body and tail. The postoperative course was uneventful and a three-year disease free survival has been achieved so far.
Figure 5 Histopathologic findings (HE × 200).
A: Histopathological examination showed the presence of cystically dilated ducts with formation of papillary growth lined by mucin-secreting columnar epithelium at the level of the pancreatic head; B: The mucin-secreting epithelium reveals stratification of atypical nuclei. No obvious stromal invasion was seen and the features suggested a diagnosis of intraductal papillary mucinous carcinoma, noninvasive type; C: The section of the pancreatic body showed that it was focally involved by the lesion (arrow); D: The section of the pancreatic tail showed the focal presence of atypical ducts.
DISCUSSION
An enormous increase in the diagnosis of IPMN has occurred in recent years[16–18], which is due both to the improvement and widespread use of modern imaging equipments and to a heightened awareness among physicians.
IPMN usually occurs in 60 to 70 years old males, most of whom have a long-standing history of recurrent acute pancreatitis or symptoms suggestive of chronic obstructive pancreatitis, similarly to our patient, due to intermittent obstruction of the pancreatic duct with mucus plugs[19]. Other symptoms are nonspecific[2021] and no signs or symptoms are pathognomonic of primary cystic neoplasms of the pancreas, including IPMN. Furthermore, many patients with no symptoms have their tumors detected incidentally at imaging studies performed for unrelated indications. Thus, there is often a several months delay in the diagnosis of IPMN, due to the insidious nature of this disease and to the lack of awareness of this entity among physicians[132022–24]. Some patients may have elevations of pancreatic enzymes with episodes of pain (with or without biliary obstruction), but in most cases routine laboratory tests are normal. Similarly, tumor markers, such as CA 19-9 and CEA, are elevated in less than one-fifth of cases only, and are not always indicative of a malignant transformation of the tumors[19]. In this case, there were normal routine laboratory tests and tumor markers.
IPMN is a precancerous lesion with a well described adenoma-carcinoma sequence. However, the rate of progression appears to be extremely slow (approximately 15 to 20 years)[25].
The IPMN has been classified as main duct type (MDT-IPMN) or branch duct type (BDT-IPMN), based upon the anatomic involvement of the pancreatic duct[2627]. The majority of IPMNs are located in the pancreatic head (75%) whereas the rest involves the body/tail regions[15]. Multifocal neoplasms have been hypothesized[15], but the real prevalence of multifocality is still unknown. The final diagnosis of this case proved the existence of this rare entity.
The majority of patients with IPMN does not have invasive cancer, and usually have a prolonged course without the development of cancer. In one series, the ten-year actuarial risk of high grade dysplasia and invasive cancer were 49% and 29%, respectively[28]. The five-year actuarial risk of progression was significantly higher in patients with main duct type compared with those with exclusively the branch duct type (63% versus 15%). Surgery is the only treatment option in patients who do have high grade dysplasia or cancer detected by endoscopic or cytological examination. Preoperative staging, using a combination of ERCP, pancreatoscopy, EUS and intraductal ultrasonography, may be beneficial to determine the indication to surgery[29–32]. However, accurate prediction of malignancy is not possible preoperatively. Sugiyama et al[33] proposed some predictive factors of malignant IPMN, including (1) tumor size, since an IPMN > 4 cm and a branch duct IPMN > 3 cm suggest malignancy; (2) a mural nodule of a size > 5 mm; (3) a dilatation of main pancreatic duct > 10 mm; (4) the patient age, the mean age for adenomas being 64, whereas that of invasive cancers is 67; and (5) symptoms, especially jaundice.
Selection of a surgical procedure for treating IPMN remains controversial, because IPMNs show a wide spectrum of histologic changes, ranging from hyperplasia to invasive carcinoma and because of the so far unresolved question of multifocality. As demonstrated in this patient, the aim of surgery is to achieve complete resection with negative margins for invasive and noninvasive disease and to minimize the incidence of recurrence in the pancreatic remnant. However, an extended resection of the pancreas may pose the risk of inducing a severe pancreatic functional insufficiency, both exocrine and endocrine.
Another difficult question is the management of patients with suspected IPMNs who do not have high grade dysplasia or cancer detected during initial evaluation, and of patients with small (< 3 cm) branch-duct IPMNs. Our opinion is that management should be based upon the confidence in the diagnosis, the presence of symptoms and a discussion with the patient regarding the possible risk of subsequent malignant transformation. Surgery can be reasonably offered to patients who are good surgical candidates in order to confirm the diagnosis and to prevent the subsequent development of cancer. In contrast, patients who are poor surgical candidates can be treated conservatively with an imaging test performed every six to twelve months to evaluate the stability of the lesion. Our case fared very well despite the fact that he was 72 years old. Originally, we suggested the resection of pancreatic head with close follow-up of the other two tumors, considering the tiny risk of malignant transformation, but both the patient and his family preferred a complete resection of the three tumors. Finally, we performed an extended Whipple operation (including the partial resection of the pancreatic body) with a distal pancreatectomy. The postoperative course was uneventful without deterioration of the diabetes and exocrine insufficiency.
The final pathologic diagnosis was noninvasive mixed (involving both the main and the branch duct) type IPMN (carcinoma in situ) affecting the pancreatic head and a branch duct type IPMN (duct atypia) of the pancreatic body and tail. This confirmed the occurrence of multifocality of IPMNs of the pancreas. The patient has until now achieved a three year disease free survival.
Peer reviewer: David L Carr-Locke, MD, Director of Endoscopy, Brigham and Women’s Hospital, Endoscopy Center, Brigham and Women’s Hospital, 75 Francis St, Boston MA 02115, United States