Published online Dec 7, 2009. doi: 10.3748/wjg.15.5654
Revised: September 14, 2009
Accepted: September 21, 2009
Published online: December 7, 2009
AIM: To examine whether shift work accelerates metabolic syndrome (MetS) development among early middle-aged males with elevated alanine aminotransferase (e-ALT).
METHODS: A retrospective, observational follow-up study on MetS development at a 5-year interval was conducted using health examination data. Nine hundred and ninety six male employees not fulfilling MetS criteria at screening were enrolled. Age, MetS-components, liver enzymes, serological markers for viral hepatitis, abdominal ultrasound, insulin resistance status, lifestyles, and workplace factors were analyzed.
RESULTS: The prevalence of elevated serum ALT (> 40 U/L, e-ALT) at baseline was 19.1%. There were 381 (38.3%) workers with long-term exposures to day-night rotating shift work (RSW). 14.2% of subjects developed MetS during follow-up. After 5 years, the workers with e-ALT had significantly unfavorable changes in MetS-components, and higher rates of MetS development, vs subjects with normal baseline ALT levels. Workers with both baseline e-ALT and 5-year persistent RSW (pRSW) exposure had the highest rate of MetS development. Also, e-ALT-plus-pRSW workers had a significant increase in MetS-components at follow-up, compared with the other subgroups. After controlling for potential confounders, e-ALT-plus-pRSW workers posed a significant risk for MetS development (odds ratio, 2.7; 95% confidence interval, 1.4-5.3, vs workers without baseline e-ALT nor pRSW).
CONCLUSION: We suggest that all early middle-aged male employees with e-ALT should be evaluated and managed for MetS. Particularly in terms of job arrangements, impacts of long-term RSW on MetS development should be assessed for all male employees having baseline e-ALT.
- Citation: Lin YC, Hsiao TJ, Chen PC. Shift work aggravates metabolic syndrome development among early-middle-aged males with elevated ALT. World J Gastroenterol 2009; 15(45): 5654-5661
- URL: https://www.wjgnet.com/1007-9327/full/v15/i45/5654.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5654
Elevated serum alanine aminotransferase (ALT) is a common abnormality of health examinations among the middle-aged working population[1,2]. Nowadays, it is unavoidable that a large number of asymptomatic workers with elevated ALT (e-ALT), regardless of the underlying cause, are asked do rotating shift work (RSW) on 24-h production lines[3]. In previous studies, e-ALT[4,5] and shift work[6,7] had been independently assessed for their associations with metabolic syndrome (MetS), which has been linked with cardiovascular disease (CVD)[8,9], one of the leading death causes among working populations[10]. However, for early middle-aged workers with e-ALT in baseline conditions, few long-term follow-up studies assessed the association between RSW and MetS development. In terms of workplace health management and job arrangement, surveys for the impact of RSW on MetS development among the subjects having e-ALT urgently required. In Taiwan, periodic health checkups are compulsorily for employees in many workplaces; thus we had the opportunity to utilize a retrospective follow-up study to assess the impact of RSW on MetS development among early-middle-aged workers having baseline e-ALT.
In accordance with the Labor Health Protection Regulation of the Labor Safety and Health Act, 1203 male workers of an electronic manufacturing company underwent compulsory health checkups in 2002 and 2007. Final analysis of this follow-up study only included the subjects not fulfilling MetS criteria in 2002. Among the 1203 workers undergoing health examinations in both 2002 and 2007, 207 employees were excluded from study because they were screened previously with MetS. Final records of a total of 996 male workers constituted the cohort for the study and endpoint analysis. Hepatic virus infection is highly prevalent among Taiwanese adults[2], our analysis included the hepatic virus carriers without MetS, and we controlled these factors in our final multivariate analysis. Most of the male employees of this electronics manufacturing company were residents of northern Taiwan.
The procedures and measurements of the health examinations took place in the morning, following an overnight fast, at a health-care unit of the factory. This health examination was open to all registered employees during every working day, 07:30 am to 09:30 am, for a 1-mo span. We suggested that the health checkups for the rotating shift workers be performed on the 3rd to 6th day of their day-duty. All the employees were requested to avoid drastic physical exercises (i.e. long-distance running, heavy weight lifting training etc) during the 3 d before undergoing the health examination.
Subjects’ identities were anonymous and unlinked to the data. This analytical study, limited within health checkups records, followed the ethical criteria for human research, and the study protocol (TYGH09702108) was reviewed and approved by the Ethics Committee of the Tao-Yuan General Hospital, Taiwan.
In 2002, a questionnaire about baseline personal history, including smoking habits, physical exercise, drinking, and dietary habits, was completed by the examinees. The day-night RSW was determined from the self-reported questionnaires in 2002 and 2007. The shifts on the 24 h production line were scheduled on a three-team/two-shift plan (6 d-shifts-three rest days-six night-shifts-three rest days, etc.). The day and night shifts started at 07:30 am and 19:30 pm, respectively.
Physical examination and blood tests were performed on all participants in both 2002 and 2007. The participants arrived at the health care unit in the morning, between 07:30 am and 09:30 am, after an overnight 8 h fast. The physical examination records included measurements of waist circumference, weight, height, and blood pressure. Waist circumferences were measured midway between the lowest rib and the superior border of the iliac crest. After being seated for 5 min, sitting blood pressure was measured with the dominant arm using digital automatic sphygmomanometers (model HEM 907, Omron, Japan), twice at a 5-min intervals; the mean of the reading was used for data analysis. After the physical examination, participants were placed in a reclined position, and venous blood (20 mL) from an antecubital vein of the arm was taken for subsequent tests. Blood specimens were centrifuged immediately thereafter, and shipped in the frozen state, using dry ice, to a central clinical laboratory in the Tao-Yuan General Hospital (certified by ISO 15189 and ISO 17025). Glucose [glucose oxidase method, intra- and inter-assay coefficient of variation (CV) < 5%], triglyceride (enzymatic method, intra- and inter-assay CV < 5%), high-density lipoprotein (HDL) (enzymatic method, intra- and inter-assay CV < 5%), ALT, aspartate aminotransferase (Japan Society of Clinical Chemistry method, intra- and inter-assay CV < 5%) assays were conducted by a Hitachi autoanalyzer model 7150 (Hitachi, Tokyo, Japan). hepatitis B virus (HBV) surface antigen, anti-HBV surface antibody and anti-HCV antibody were measured by the microparticle enzyme immunoassay using the AxSym System instrument (Abbott, Illinois, USA), with the intra-assay and inter-assay CV < 10%. Insulin was determined with an AxSym autoanalyzer (Abbott, Illinois, USA) radioimmunoassay, with the intra-assay and inter-assay CV < 10%.
Institutional Review Board of Tao-Yuan General Hospital did not agree to perform liver biopsies on apparently healthy subjects. Sonographic diagnosis for fatty liver is wildly accepted in many epidemiological surveys[11]; therefore, we used this noninvasive method to diagnose fatty liver. In 2002, abdominal ultrasound examinations were performed using convex-type real-time electronic scanners (Toshiba SSA-340 with 3.75 MHz convex-type transducer) by three gastrointestinal specialists. These gastrointestinal specialists had 10-15 years of experience in abdominal ultrasound examinations, and were blind to the medical history as well as to the blood test results of the examinees.
MetS: The pre-MetS status was defined as one or two[12], and the MetS designation was made if three or more of the following five criteria were fulfilled: Central obesity (waist circumference > 90 cm based on Taiwanese criteria[13]); Elevated blood pressure (defined as systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 85 mmHg; Hyperglycemia, hypo-HDL cholesterolemia and hypertriglyceridemia (defined based on modified National Cholesterol Education Program Adult Treatment Panel III criteria[14] as: fasting sugar ≥ 100 mg/dL, HDL < 40 mg/dL, and triglycerides ≥ 150 mg/dL). We specified the subjects with MetS outcome as those who were without MetS in 2002 but with MetS in 2007.
e-ALT: e-ALT was defined as ALT > 40 U/mL, according to the standard reference limits used at Tao-Yuan General Hospital and other by studies[15].
Insulin resistance: Insulin resistance was calculated by the equation of homeostasis model assessment, HOMAIR = fasting insulin (U/mL) × glucose (mmol/L)/22.5, and defined as the top quartile of HOMAIR[16].
Persistent RSW (pRSW): According to the Labor Health Protection Regulation of the Labor Safety and Health Act, periodical health examination ought to be performed on all registered employees working more than 1 year. These examinations took place annually for workers in this company. In the questionnaire, workers answered “yes” to “my job is a rotating shifting work”, indicating that the workers had done a rotating job for at least 1 year. Generally, this working pattern continues except for reasons of family events or promotions in this kind of factory. Those who did RSW for at least 1 year in the beginning and at the end of our follow-up were likely to have continued doing the same types of jobs. For the reasons mentioned above, we arbitrarily defined in our analysis of job type as: “pRSW” exposure was defined when the answers to the question in 2002 and 2007 were both day-night rotating shift work. Work information obtained through a self-administered questionnaire has been generally accepted in previous investigations[6,17].
Lifestyle factors: For defining “ever been a smoker”, the question started with “Do you smoke? (1 Never, 2 Currently smoke, 3 Did but quit now)” and was followed by questions about quantity. We defined “ever been a smoker” as saying “yes” to question 2 or 3 and consuming at least 10 cigarettes daily for over 1 year. For defining “used to having snacks”, the question started with “Are you used to having snacks before sleeping?” and “Are you used to having snacks between meals?” and followed with questions about quantity. We defined the habits of having snacks in the present study as more than three times per week. The standard portions of examples for snacks (fruit products, milk products, fried food, nuts, beans products, meat, and alternatives) were explained to examinees in questionnaires. We defined “having routine physical exercise” as answering “doing exercises more than three times every week”. For defining “habitual drinker”, the question started with “Do you consume alcohol as usual? (1 No, 2 Yes)” and was followed by questions about frequency and quantity. We defined “habitual drinker” in this study as saying “yes” to question 2 and consuming it at least once per week for over 1 year.
Sonographic fatty liver: The definition of ultrasonic fatty liver was based on a comparative assessment of image brightness relative to the kidneys, under identical criteria[18].
Baseline characteristics and abnormal rates were compared between four subgroups divided by e-ALT and pRSW using ANOVA and Tukey’s test for continual variables and χ2 test for categorical variables. Modes and ranges represented the initial, the 5th year and the changes of MetS-component numbers, were also tested by ANOVA and Tukey’s test. Baseline and the 5th year abnormality rates were compared using the χ2 test. Multivariate logistic regression, adjusted for age, baseline insulin resistance status, MetS-components, pRSW exposure, exercise, smoking, drinking and diet behaviors, viral hepatitis infection, and fatty liver was used to estimate the adjusted odds ratios (OR) and 95% confidence intervals (CI) of risk factors for predicting MetS development. A two-sided P-value < 0.05 was considered statistically significant. SAS version 8.0 (SAS Institute, Cary, NC, USA) was used for all statistical analyses. Excel 2000 (Microsoft®, USA) was used for the statistical figure.
Job descriptions are shown in Table 1. According to the characteristics of automatic production lines in such factories, most employees do light manual labor jobs or sedentary work. Tables 2 and 3 present baseline data of the overall and the four subgroups divided by initial e-ALT and pRSW exposures. Our sample population had an initial mean age of 32.1 years (SD 5.9 years); with total prevalence rates of e-ALT and pRSW of 19.1% and 38.3%, respectively. There were 14.2% (141/996) subjects who developed MetS within 5 years. At baseline, there were significant differences in age among the four subgroups; the subgroups with pRSW exposures were younger than the other two subgroups without pRSW exposures (30.9 and 31.4 years vs 32.8 and 32.6 years, Table 2). Baseline measurements of MetS-components, liver enzymes and insulin were significantly unfavorable in the two subgroups with e-ALT (Table 2). As shown in Table 3, baseline abnormality rates of MetS-components, hepatic virus infections, insulin resistance, and fatty liver were higher in the two groups with baseline e-ALT than in the subgroups with normal baseline ALT, though the baseline prevalence rates of central obesity and hyperglycemia were not significantly different among the four groups. Having snacks before bedtime and smoking habits prevailed in the group of pRSW with normal ALT, whereas other lifestyle parameters were not significantly different among the four groups. The overwhelming majority of pRSW workers (92.4%) were on-site workers (Tables 1 and 3).
| Job title | Routine work activities | n = 996 | e-ALT: no; pRSW: no (n = 496) | e-ALT: no; pRSW: yes (n = 310) | e-ALT: yes; pRSW: no (n = 119) | e-ALT: yes; pRSW: yes (n = 71) |
| Operator | On-site work | 232 (23.3) | 101 | 97 | 17 | 17 |
| Technician | On-site work | 422 (42.4) | 158 | 178 | 40 | 46 |
| Engineer | On-site work | 159 (16.0) | 131 | 2 | 24 | 2 |
| Team leader | On-site work | 85 (8.5) | 33 | 29 | 18 | 5 |
| Specialist | On-site work | 22 (2.2) | 16 | 1 | 5 | 0 |
| Deputy manager | Management | 42 (4.2) | 31 | 1 | 10 | 0 |
| General manager | Management | 19 (1.9) | 15 | 0 | 4 | 0 |
| Director | Management | 3 (0.3) | 3 | 0 | 0 | 0 |
| Guard | Security | 8 (0.8) | 6 | 1 | 1 | 0 |
| Clerk | Office work | 4 (0.4) | 2 | 1 | 0 | 1 |
| Measurement (SD) | Total population (n = 996) | e-ALT: no; pRSW: no (n = 496) | e-ALT: no; pRSW: yes (n = 310) | e-ALT: yes; pRSW: no (n = 119) | e-ALT: yes; pRSW: yes (n = 71) | P value2 |
| Age (yr) | 32.1 (5.9) | 32.8 (6.0) | 30.9 (5.8) | 32.6 (5.3) | 31.4 (5.7) | 0.0001 |
| Body mass index (kg/m2) | 23.4 (2.9) | 23.2 (2.8) | 22.7 (2.8) | 25.0 (2.5) | 25.0 (3.0) | < 0.0001 |
| Waist (cm) | 78.3 (7.6) | 78.1 (7.4) | 76.4 (7.4) | 82.5 (6.3) | 81.3 (8.1) | < 0.0001 |
| Systolic blood pressure (mmHg) | 119.2 (14.1) | 118.2 (13.8) | 118.2 (14.3) | 123.0 (14.0) | 123.5 (14.1) | 0.0002 |
| Diastolic blood pressure (mmHg) | 72.9 (9.0) | 72.3 (8.6) | 72.3 (8.9) | 75.4 (10.0) | 75.7 (9.3) | 0.0002 |
| Fasting blood glucose (mg/dL) | 94.5 (14.8) | 95.2 (18.6) | 92.9 (8.1) | 95.1 (8.1) | 94.5 (15.2) | 0.1797 |
| Triglycerides (mg/dL) | 113.1 (86.3) | 109.3 (97.8) | 101.0 (55.6) | 149.3 (96.0) | 132.2 (74.7) | < 0.0001 |
| HDL cholesterol (mg/dL) | 48.6 (11.0) | 49.3 (10.9) | 49.6 (11.4) | 45.2 (10.8) | 44.9 (7.9) | < 0.0001 |
| Alanine aminotransferase (U/L) | 31.0 (29.4) | 21.7 (7.7) | 21.1 (8.4) | 68.8 (42.6) | 75.8 (54.3) | < 0.0001 |
| Aspartate aminotransferase (U/L) | 25.8 (17.9) | 21.9 (5.1) | 21.8 (5.5) | 39.3 (23.3) | 48.5 (47.8) | < 0.0001 |
| Insulin (mg/dL) | 8.5 (6.2) | 8.0 (4.9) | 7.4 (5.2) | 11.4 (7.8) | 11.6 (11.4) | < 0.0001 |
| Abnormalities | Total population (n = 996) | e-ALT: no; pRSW: no (n = 496) | e-ALT: no; pRSW: yes (n = 310) | e-ALT: yes; pRSW: no (n = 119) | e-ALT: yes; pRSW: yes (n = 71) | P value2 |
| MetS-component abnormality (%) | ||||||
| Central obesity | 3.6 (36/996) | 2.8 | 3.2 | 5.9 | 7.0 | 0.1595 |
| Hyperglycemia | 20.7 (206/996) | 22.0 | 18.4 | 22.7 | 18.3 | 0.5658 |
| Hypertriglyceridemia | 17.5 (174/996) | 15.1 | 13.2 | 34.5 | 23.9 | < 0.0001 |
| Elevated blood pressure | 21.3 (212/996) | 18.3 | 20.6 | 29.4 | 31.0 | 0.01 |
| Hypo-HDL cholesterol | 20.2 (201/996) | 18.5 | 17.1 | 32.8 | 23.9 | 0.0018 |
| Elevated alanine aminotransferase | 19.1 (190/996) | |||||
| Hepatovirus B carrier | 19.3 (192/996) | 18.1 | 14.8 | 27.7 | 32.4 | 0.0005 |
| Hepatovirus C carrier | 1.2 (12/996) | 0.4 | 0.3 | 4.2 | 5.6 | < 0.0001 |
| Fatty Liver | 30.8 (307/996) | 24.0 | 21.0 | 66.4 | 62.0 | < 0.0001 |
| Lifestyle factor (%) | ||||||
| Ever been a smoker (≥ 1 yr) | 42.7 (425/996) | 36.9 | 51.6 | 39.5 | 49.3 | 0.0003 |
| Having snacks before sleeping (≥ 1 d/wk) | 42.5 (423/996) | 37.7 | 51.9 | 39.5 | 39.4 | 0.0008 |
| Having snacks between meals (≥ 1 d/wk) | 40.9 (407/996) | 40.5 | 39.4 | 47.1 | 39.4 | 0.5192 |
| Physical exercise (≥ 3 times/wk) | 34.6 (345/996) | 38.5 | 31.6 | 31.9 | 25.4 | 0.0545 |
| Habitual drinker (≥ 1 d/wk) | 10 (99/996) | 8.7 | 10.6 | 10.1 | 15.5 | 0.3196 |
| Workplace factor (%) | ||||||
| Persistent day-night rotating shift work | 38.3 (381/996) | |||||
| On-site worker | 92.4 (920/996) | 88.5 | 99.1 | 87.4 | 98.6 | < 0.0001 |
| Development of MetS within 5 yr | 14.2 (141/996) | 11.1 | 8.7 | 27.7 | 36.6 | < 0.0001 |
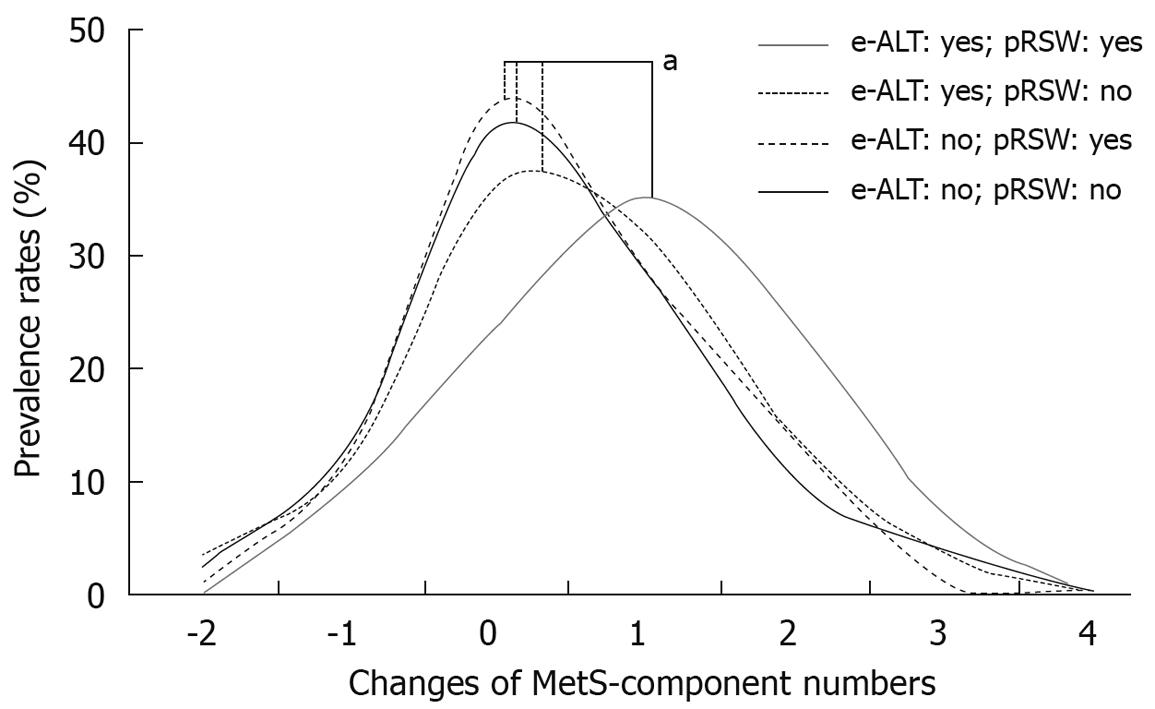
Most of the 5th year and 5-year changes of abnormality rates for MetS-component were unfavorable in the two subgroups with baseline e-ALT, and particularly in the subgroup with e-ALT-plus-pRSW (Table 4). The male workers having both baseline e-ALT and 5-year exposures to pRSW had the highest increasing rates of hyperglycemia (15.5% increased), hypertriglyceridemia (21.1% increased) and elevated blood pressure (31.0% increased). They also displayed the highest rate of MetS development (36.6%) among four subgroups. As shown on figure, the subgroup of e-ALT-plus-pRSW had a significantly increasing shift in numbers of MetS-component.
| MetS and its components | e-ALT initially | pRSW exposure | Baseline | P value1 | 5th yr | P value1 | Δ 5th yr vs baseline |
| Central obesity | No | No | 2.8 | 0.1595 | 22.2 | 0.0037 | 19.4 |
| Yes | 3.2 | 20.0 | 16.8 | ||||
| Yes | No | 5.9 | 33.6 | 27.7 | |||
| Yes | 7.0 | 33.8 | 26.8 | ||||
| Hyperglycemia | No | No | 22.0 | 0.5658 | 16.7 | < 0.0001 | -5.2 |
| Yes | 18.4 | 13.9 | -4.5 | ||||
| Yes | No | 22.7 | 26.9 | 4.2 | |||
| Yes | 18.3 | 33.8 | 15.5 | ||||
| Hypertriglyceridemia | No | No | 15.1 | < 0.0001 | 26.8 | < 0.0001 | 11.7 |
| Yes | 13.2 | 25.5 | 12.3 | ||||
| Yes | No | 34.5 | 42.0 | 7.6 | |||
| Yes | 23.9 | 45.1 | 21.1 | ||||
| Elevated blood pressure | No | No | 18.3 | 0.01 | 48.0 | 0.1025 | 29.6 |
| Yes | 20.6 | 50.0 | 29.4 | ||||
| Yes | No | 29.4 | 55.5 | 26.1 | |||
| Yes | 31.0 | 62.0 | 31.0 | ||||
| Hypo-HDL cholesterol | No | No | 18.5 | 0.0018 | 6.3 | < 0.0001 | -12.3 |
| Yes | 17.1 | 8.1 | -9.0 | ||||
| Yes | No | 32.8 | 17.6 | -15.1 | |||
| Yes | 23.9 | 23.9 | 0.0 | ||||
| Development of MetS within 5 yr | No | No | 11.1 | < 0.0001 | |||
| Yes | 8.7 | ||||||
| Yes | No | 27.7 | |||||
| Yes | 36.6 | ||||||
| MetS-component numbers (mode) | No | No | 0 | 1 | 0 | ||
| Yes | 0 | 1 | 0 | ||||
| Yes | No | 2 | 1 | 0 | |||
| Yes | 1 | 2 | 1 |
Table 5 shows the age-adjusted OR for MetS obtained by the step-by-step examinations. At the beginning, the age-adjusted OR of MetS development for the e-ALT workers without or with pRSW exposure were 3.1 (95% CI: 1.9-5.0) and 4.7 (95% CI: 2.7-8.2) respectively. Combined with step by step examinations, after controlling for confounders of initial age, MetS-components, insulin resistance, liver status, lifestyle and workplace factors, the multivariate analysis indicated that the male workers with baseline e-ALT plus pRSW remained at a 2.7-fold (95% CI: 1.4-5.3) greater risk of developing MetS compared with those having neither initial e-ALT nor pRSW exposure. Male employees who had initial e-ALT but without pRSW had an increased risk for progression to MetS when compared with those who initially had normal ALT. This result was significant but became insignificant when fatty liver was introduced as one of the controlling factors: adjusted OR of MetS development became to 1.3 (95% CI: 0.7-2.3) for the workers having initial e-ALT but without pRSW (Table 5).
| Adjustment for initial characteristics | OR1 (e-ALT: no vs pRSW: no) | 95% CI | ||
| Model 1 | Age (yr) | e-ALT: no; pRSW: yes | 0.8 | 0.5-1.3 |
| e-ALT: yes; pRSW: no | 3.1 | 1.9-5.0 | ||
| e-ALT: yes; pRSW: yes | 4.7 | 2.7-8.2 | ||
| Model 2 | Age, MetS-components, insulin resistance | e-ALT: no; pRSW: yes | 0.8 | 0.5-1.3 |
| e-ALT: yes; pRSW: no | 1.8 | 1.03-3.0 | ||
| e-ALT: yes; pRSW: yes | 3.8 | 2.0-6.9 | ||
| Model 3 | Age, MetS-components, insulin resistance, hepatovirus infections | e-ALT: no; pRSW: yes | 0.8 | 0.5-1.3 |
| e-ALT: yes; pRSW: no | 1.9 | 1.1-3.2 | ||
| e-ALT: yes; pRSW: yes | 4.0 | 2.1-7.4 | ||
| Model 4 | Age, MetS-components, insulin resistance, hepatovirus infections, fatty liver | e-ALT: no; pRSW: yes | 0.8 | 0.5-1.3 |
| e-ALT: yes; pRSW: no | 1.4 | 0.8-2.4 | ||
| e-ALT: yes; pRSW: yes | 2.9 | 1.5-5.5 | ||
| Model 5 | Age, MetS-components, insulin resistance, hepatovirus infections, fatty liver, lifestyle and workplace factors | e-ALT: no; pRSW: yes | 0.7 | 0.4-1.2 |
| e-ALT: yes; pRSW: no | 1.3 | 0.7-2.3 | ||
| e-ALT: yes; pRSW: yes | 2.7 | 1.4-5.3 | ||
All the subjects in our sample population continued working in the company at least for 5 years, which reveals that our workers were in relatively stable occupational situations. Our conclusions from observations of a large and stable middle-aged working population will contribute to the surveys on MetS in such working groups.
The baseline e-ALT prevalence rate (19.1%) in our sample population was as high as that in other working populations[1]. Although not fulfilling MetS criteria at screening, the workers with e-ALT had significantly more unfavorable baseline measurements, as well as higher abnormality rates for most of the MetS-components (Tables 2 and 3), as compared with workers having normal baseline ALT. Similar findings were revealed in a previous cross-sectional study for young healthy men[19]. Our follow-up results (Table 4) demonstrated that the subjects initially having e-ALT tended display considerably raised abnormality rates of MetS-components within 5 years. Moreover, at the end of follow-up, the two subgroups with baseline e-ALT had significantly higher rates of MetS development than the other two subgroups with normal baseline ALT levels.
As a mortality predictor[20], e-ALT represents many detrimental health conditions, including systemic inflammation[19,20], which was reported to contribute to MetS development. In our step-by-step multivariate analysis (Table 5), the significant impacts of baseline e-ALT on MetS development among the workers without pRSW remained until we introduced an operator-dependent survey, fatty liver[21], which has an extremely close relationship with e-ALT[15], as a controlling factor. According to our statistical analysis, baseline e-ALT is associated with long-term development of manifold metabolic abnormalities and MetS among early middle-aged male workers. We suggest workplace MetS managements for all workers having e-ALT.
Taking each MetS-component into consideration, most of the MetS-components abnormality rates increased as our population got older (Table 4); though some exceptions for hyperglycemia and hypo-HDL cholesterolemia were found in the subgroups with normal baseline ALT or without pRSW exposure.
We demonstrated that the early middle-aged male workers with baseline e-ALT had increased abnormality rates of hyperglycemia, same as previous findings[22]. Similarly, a significantly increased prevalence of hyperglycemia was found for workers with e-ALT-plus-pRSW, which was compatible with the findings that shift work can aggravate the insulin resistance[23]. In contrast, for the workers with normal baseline ALT levels, hyperglycemia showed an improving trend. These phenomena of improved blood sugar were dissimilar to the elderly population[24], but have been shown in many follow-up observations for healthy adults[22,25]. Our early middle-age subjects with normal baseline ALT seemed to maintain the ability to stabilize blood sugar. In contrast, the subjects with baseline e-ALT displayed impaired abilities to improve their blood sugar levels, which might be related to pRSW exposure. Also, hypo-HDL was associated with an improving trend among our early-middle-aged males during follow-up, except for the e-ATL-plus-pRSW workers. This increasing trend of HDL has been mentioned among healthy adults[26], but not found in the elderly[24]. As apparently healthy young adults get older, HDL might quantitatively increase in response to external stimuli[24,27] or internal challenges[28], and this increase is likely to reach a plateau[29]. Our comparatively young healthy workers might not yet have reached their plateau, so they might have increasing HDL concentrations within our follow-up. However, when under chronic oxidative stress caused by simultaneous e-ALT[30] and shift work exposure[31], our early middle-aged subjects might have significantly weaker capabilities for quantitatively increasing HDL.
Analyzing the number of MetS-components is informative in determining the CVD risk of the general population[9]. In addition, MetS-component numbers were reported in many convincing studies that closely linked them with damaged conditions of the whole human body: impaired cardiovascular conditions[32,33], cognitive impairment[34], and colon polyps[35]. Shift work is associated with the premature aging process[36], and as demonstrated in our analyses (Table 4 and Figure 1), the e-ATL-plus-pRSW workers had significantly increased numbers of MetS-components in the end of follow-up. Thus, the impacts of shift work exposure on general health conditions of the e-ALT workers should receive close attention.
Some factors might confound our findings, such as: high prevalence of HBV and HCV among those with e-ALT at baseline, fatty liver might be linked with HCV carrier status, and the reduction in the amount of exercise in individuals who developed MetS. However, in the analysis carried out in consecutive steps for the male workers with e-ALT, all the other confounders did not remove the significance of pRSW on MetS development (Table 5, Model 3, 4 and 5). Our follow-up observations confirmed that persistent day-night RSW accelerates the progression toward MetS among the e-ALT workers. e-ALT can work with other risk factors to worsen the inflammatory state[30], and shift work brings about long-term oxidative stress[31]; therefore a possible explanation for our male shift workers with e-ALT tending to develop MetS might be aggravated systemic chronic inflammatory reactions[37,38].
Due to the dramatically rising occurrence rate of MetS, we strongly suggest screening, follow-up, and treatment of MetS for workers with both e-ALT and unavoidable long-term rotational shift work exposures.
Some potential limitations of our analysis need to be considered.
Firstly, Taiwan National Health Insurance has provided comprehensive medical care, and for the pre-MetS subjects, it was possible that they could have had corrective management during our follow-up, and those actions might have led to protective effects[39]. In such a case, the main limitations of this observational investigation would be that we did not take into consideration the potential treatment effects for the at risk workers. Thus, our conclusion might be affected by the results of therapeutic management programs, and we might be underestimating risk factors for MetS development. Secondarily, our shift workers with normal ALT were relatively young in our sample population (Table 2), as compared with workplace populations of other studies[6]. Physically, the advantage of youthfulness[6] might lead us to a statistically insignificant conclusion for the shift work exposures on MetS development among subjects with normal ALT (Table 5); the aging effect on MetS development is an important issue for such a young cohort, and requires further surveys. Lastly, we proposed that MetS development among our e-ALT shift workers might be closely linked to chronic inflammation; therefore direct inflammatory evidence[19] should be carefully surveyed in future investigations.
As for the increasing body of professional women occupying workplaces, it is important to compare the differences between genders in risk assessments for MetS development; thus all the present findings are worthy of being tested in future studies of female workers. In terms of health and safety, for the individuals who initially had MetS and were excluded of this study, it is necessary to assess their cardiovascular outcomes in future investigations.
In conclusion, for early middle-aged male workers, baseline and changes of MetS component abnormalities, as well as the development of MetS, are all associated with baseline e-ALT. In addition, long-term RSW exposures significantly aggravate MetS development among the workers having baseline e-ALT. We suggest all male workers having e-ALT should be carefully evaluated and managed for MetS. Particularly in terms of job arrangements, impacts of long-term RSW on MetS development should be assessed for all male employees having baseline e-ALT.
Elevated serum alanine aminotransferase (ALT) is a common abnormality of health examinations among middle-aged working populations. It is unavoidable nowadays that a large number of asymptomatic workers with elevated serum ALT levels might be asked to do rotating shift work (RSW) on 24-h production lines. In terms of workplace health management and job assignment, a 5-year follow-up study assessing the association between RSW and metabolic syndrome (MetS) development was conducted in Taiwan for male workers.
In some studies, elevated ALT (e-ALT) and shift work had been independently assessed for their associations with MetS, which is associated with cardiovascular disease, one of the leading causes of death among working populations. This survey takes these two risk factors together into consideration and obtained significant findings.
Workers who had both baseline e-ALT and persistent RSW (pRSW) exposures (e-ALT-plus-pRSW workers) had a significantly high risk for MetS development among middle-aged male workers. In addition, e-ALT-plus-pRSW workers had a significant increase in MetS-component numbers at the end of follow-up, compared with the other workers. Finally, e-ALT-plus-pRSW workers had a significant risk for MetS development.
All the workers with e-ALT should be carefully evaluated and managed for MetS. Particularly, MetS risk assessment must be emphasized for male employees having e-ALT and facing long-term RSW exposures.
The public health expert reviewers agreed the important area of research given the amount of shift work performed around the globe; they also pointed out that, given the large number of individual records and individuals examined, this is an important 5 years retrospective analysis of associated factors.
Peer reviewer: Wing-Kin Syn, MBChB (Honours), MRCP (UK), Division of Gastroenterology, GSRB-1, Suite 1073, DUMC 3256, 595 LaSalle Street, Durham, NC 27710, United States
S- Editor Li LF L- Editor Stewart GJ E- Editor Zheng XM
| 1. | Nakamura K, Motohashi Y, Kikuchi S, Tanaka M, Nakano S. Liver transferase activity in healthy Japanese employees aged 18-39 years. Ind Health. 1998;36:218-222. [Cited in This Article: ] |
| 2. | Lin YC, Hsiao ST, Chen JD. Sonographic fatty liver and hepatitis B virus carrier status: synergistic effect on liver damage in Taiwanese adults. World J Gastroenterol. 2007;13:1805-1810. [Cited in This Article: ] |
| 3. | Su SB, Lu CW, Kao YY, Guo HR. Effects of 12-hour rotating shifts on menstrual cycles of photoelectronic workers in Taiwan. Chronobiol Int. 2008;25:237-248. [Cited in This Article: ] |
| 4. | Goessling W, Massaro JM, Vasan RS, D'Agostino RB Sr, Ellison RC, Fox CS. Aminotransferase levels and 20-year risk of metabolic syndrome, diabetes, and cardiovascular disease. Gastroenterology. 2008;135:1935-1944, 1944.e1. [Cited in This Article: ] |
| 5. | Schindhelm RK, Dekker JM, Nijpels G, Stehouwer CD, Bouter LM, Heine RJ, Diamant M. Alanine aminotransferase and the 6-year risk of the metabolic syndrome in Caucasian men and women: the Hoorn Study. Diabet Med. 2007;24:430-435. [Cited in This Article: ] |
| 6. | De Bacquer D, Van Risseghem M, Clays E, Kittel F, De Backer G, Braeckman L. Rotating shift work and the metabolic syndrome: a prospective study. Int J Epidemiol. 2009;38:848-854. [Cited in This Article: ] |
| 7. | Ha M, Park J. Shiftwork and metabolic risk factors of cardiovascular disease. J Occup Health. 2005;47:89-95. [Cited in This Article: ] |
| 8. | Saito I, Iso H, Kokubo Y, Inoue M, Tsugane S. Metabolic syndrome and all-cause and cardiovascular disease mortality: Japan Public Health Center-based Prospective (JPHC) Study. Circ J. 2009;73:878-884. [Cited in This Article: ] |
| 9. | Knuiman MW, Hung J, Divitini ML, Davis TM, Beilby JP. Utility of the metabolic syndrome and its components in the prediction of incident cardiovascular disease: a prospective cohort study. Eur J Cardiovasc Prev Rehabil. 2009;16:235-241. [Cited in This Article: ] |
| 10. | Cheng TY, Wen SF, Astor BC, Tao XG, Samet JM, Wen CP. Mortality risks for all causes and cardiovascular diseases and reduced GFR in a middle-aged working population in Taiwan. Am J Kidney Dis. 2008;52:1051-1060. [Cited in This Article: ] |
| 11. | Mishra P, Younossi ZM. Abdominal ultrasound for diagnosis of nonalcoholic fatty liver disease (NAFLD). Am J Gastroenterol. 2007;102:2716-2717. [Cited in This Article: ] |
| 12. | Dimitrijevic-Sreckovic V, Colak E, Djordjevic P, Gostiljac D, Sreckovic B, Popovic S, Canovic F, Ilic M, Obrenovic R, Vukcevic V. Prothrombogenic factors and reduced antioxidative defense in children and adolescents with pre-metabolic and metabolic syndrome. Clin Chem Lab Med. 2007;45:1140-1144. [Cited in This Article: ] |
| 14. | Tan CE, Ma S, Wai D, Chew SK, Tai ES. Can we apply the National Cholesterol Education Program Adult Treatment Panel definition of the metabolic syndrome to Asians? Diabetes Care. 2004;27:1182-1186. [Cited in This Article: ] |
| 15. | Yoo J, Lee S, Kim K, Yoo S, Sung E, Yim J. Relationship between insulin resistance and serum alanine aminotransferase as a surrogate of NAFLD (nonalcoholic fatty liver disease) in obese Korean children. Diabetes Res Clin Pract. 2008;81:321-326. [Cited in This Article: ] |
| 16. | Lin YC, Chen JD. Association between sonographic fatty liver and ischemic electrocardiogram among non-obese Taiwanese nale adults. J Med Ultrasound. 2006;14:58-64. [Cited in This Article: ] |
| 17. | Lin YC, Hsiao TJ, Chen PC. Persistent rotating shift-work exposure accelerates development of metabolic syndrome among middle-aged female employees: a five-year follow-up. Chronobiol Int. 2009;26:740-755. [Cited in This Article: ] |
| 18. | Hsiao TJ, Chen JC, Wang JD. Insulin resistance and ferritin as major determinants of nonalcoholic fatty liver disease in apparently healthy obese patients. Int J Obes Relat Metab Disord. 2004;28:167-172. [Cited in This Article: ] |
| 19. | Kazumi T, Kawaguchi A, Hirano T, Yoshino G. Serum alanine aminotransferase is associated with serum adiponectin, C-reactive protein and apolipoprotein B in young healthy men. Horm Metab Res. 2006;38:119-124. [Cited in This Article: ] |
| 20. | Vento S, Nobili V. Aminotransferases as predictors of mortality. Lancet. 2008;371:1822-1823. [Cited in This Article: ] |
| 21. | Strauss S, Gavish E, Gottlieb P, Katsnelson L. Interobserver and intraobserver variability in the sonographic assessment of fatty liver. AJR Am J Roentgenol. 2007;189:W320-W323. [Cited in This Article: ] |
| 22. | Festa A, Williams K, D'Agostino R Jr, Wagenknecht LE, Haffner SM. The natural course of beta-cell function in nondiabetic and diabetic individuals: the Insulin Resistance Atherosclerosis Study. Diabetes. 2006;55:1114-1120. [Cited in This Article: ] |
| 23. | Nagaya T, Yoshida H, Takahashi H, Kawai M. Markers of insulin resistance in day and shift workers aged 30-59 years. Int Arch Occup Environ Health. 2002;75:562-568. [Cited in This Article: ] |
| 24. | Wilson PW, Anderson KM, Harris T, Kannel WB, Castelli WP. Determinants of change in total cholesterol and HDL-C with age: the Framingham Study. J Gerontol. 1994;49:M252-M257. [Cited in This Article: ] |
| 25. | Park YW, Chang Y, Sung KC, Ryu S, Sung E, Kim WS. The sequential changes in the fasting plasma glucose levels within normoglycemic range predict type 2 diabetes in healthy, young men. Diabetes Res Clin Pract. 2006;73:329-335. [Cited in This Article: ] |
| 26. | Fukuda H, Haruyama Y, Nakade M, Muto T. Relationship between lifestyle and change of cardiovascular risk factors based on a five-year follow-up of employees in Japan. Ind Health. 2007;45:56-61. [Cited in This Article: ] |
| 27. | Ullrich IH, Reid CM, Yeater RA. Increased HDL-cholesterol levels with a weight lifting program. South Med J. 1987;80:328-331. [Cited in This Article: ] |
| 28. | Starr JM, Shiels PG, Harris SE, Pattie A, Pearce MS, Relton CL, Deary IJ. Oxidative stress, telomere length and biomarkers of physical aging in a cohort aged 79 years from the 1932 Scottish Mental Survey. Mech Ageing Dev. 2008;129:745-751. [Cited in This Article: ] |
| 29. | Higuchi M, Iwaoka K, Fuchi T, Kobayashi S, Tamai T, Takai H, Nakai T. Relation of running distance to plasma HDL-cholesterol level in middle-aged male runners. Clin Physiol. 1989;9:121-130. [Cited in This Article: ] |
| 30. | Yamada J, Tomiyama H, Yambe M, Koji Y, Motobe K, Shiina K, Yamamoto Y, Yamashina A. Elevated serum levels of alanine aminotransferase and gamma glutamyltransferase are markers of inflammation and oxidative stress independent of the metabolic syndrome. Atherosclerosis. 2006;189:198-205. [Cited in This Article: ] |
| 31. | Ishihara I, Nakano M, Ikushima M, Hara Y, Yoshimine T, Haraga M, Nakatani J, Kawamoto R, Kasai H. Effect of work conditions and work environments on the formation of 8-OH-dG in nurses and non-nurse female workers. J UOEH. 2008;30:293-308. [Cited in This Article: ] |
| 32. | Katakami N, Kaneto H, Matsuhisa M, Umayahara Y, Kosugi K, Yamasaki Y. Clustering of several cardiovascular risk factors affects tissue characteristics of the carotid artery. Atherosclerosis. 2008;198:208-213. [Cited in This Article: ] |
| 33. | Doi Y, Ninomiya T, Hata J, Yonemoto K, Arima H, Kubo M, Tanizaki Y, Iwase M, Iida M, Kiyohara Y. Proposed criteria for metabolic syndrome in Japanese based on prospective evidence: the Hisayama study. Stroke. 2009;40:1187-1194. [Cited in This Article: ] |
| 34. | Yaffe K, Weston AL, Blackwell T, Krueger KA. The metabolic syndrome and development of cognitive impairment among older women. Arch Neurol. 2009;66:324-328. [Cited in This Article: ] |
| 35. | Lee GE, Park HS, Yun KE, Jun SH, Kim HK, Cho SI, Kim JH. Association between BMI and metabolic syndrome and adenomatous colonic polyps in Korean men. Obesity (Silver Spring). 2008;16:1434-1439. [Cited in This Article: ] |
| 36. | Kulikov VY, Fridman YM, Fomin AN. Role of oxidative stress in mechanisms of premature aging in shift labor workers. Alaska Med. 2007;49:81-84. [Cited in This Article: ] |
| 37. | Zuliani G, Volpato S, Galvani M, Blè A, Bandinelli S, Corsi AM, Lauretani F, Maggio M, Guralnik JM, Fellin R. Elevated C-reactive protein levels and metabolic syndrome in the elderly: The role of central obesity data from the InChianti study. Atherosclerosis. 2009;203:626-632. [Cited in This Article: ] |
| 38. | Kressel G, Trunz B, Bub A, Hülsmann O, Wolters M, Lichtinghagen R, Stichtenoth DO, Hahn A. Systemic and vascular markers of inflammation in relation to metabolic syndrome and insulin resistance in adults with elevated atherosclerosis risk. Atherosclerosis. 2009;202:263-271. [Cited in This Article: ] |
| 39. | Allen P, Thompson JL, Herman CJ, Whyte AN, Wolfe VK, Qualls C, Helitzer DL. Impact of periodic follow-up testing among urban American Indian women with impaired fasting glucose. Prev Chronic Dis. 2008;5:A76. [Cited in This Article: ] |