Published online Sep 14, 2009. doi: 10.3748/wjg.15.4316
Revised: August 1, 2009
Accepted: August 8, 2009
Published online: September 14, 2009
AIM: To investigate the expression of thymidylate synthase (TS) and glutathione-s-transferase π (GST-π) in esophageal squamous cell carcinoma and their association with the clinicopathologic characteristics.
METHODS: Immunohistochemical methods were used to detect the expression of TS and GST-π in surgically resected formalin-fixed, paraffin-embedded esophageal squamous cell carcinoma (ESCC) tissue sections from 102 patients (median age, 58 years) and in 28 normal esophageal mucosa (NEM) samples. The relationship between TS and GST-π expression and clinicopathologic factors was examined.
RESULTS: The expression of TS and GST-π was not statistically significantly associated with age of the patients, tumor size, lymph node metastasis, depth of invasion or tumor stage. TS staining was positive in 17.86% of normal esophageal mucosa and in 42.16% of ESCC samples (P < 0.05). The expression level of TS was not only significantly lower in well-differentiated (21.88%) than in poorly-differentiated carcinomas (51.43%, P < 0.05), but was also significantly higher in samples from male patients (46.51%) than from female patients (18.75%, P < 0.05). GST-π was positively stained in 78.57% of normal esophageal mucosa and in 53.92% of ESCC samples (P < 0.05). The expression level of GST-π was also significantly higher in well-differentiated carcinomas (65.63%) than in poorly-differentiated carcinomas (35.00%, P < 0.05).
CONCLUSION: The expression of TS and of GST-π may be used as molecular markers for the characterization of ESCC. Poorly-differentiated cells showed increased expression of TS and reduced expression of GST-π.
- Citation: Huang JX, Li FY, Xiao W, Song ZX, Qian RY, Chen P, Salminen E. Expression of thymidylate synthase and glutathione-s-transferase π in patients with esophageal squamous cell carcinoma. World J Gastroenterol 2009; 15(34): 4316-4321
- URL: https://www.wjgnet.com/1007-9327/full/v15/i34/4316.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4316
| Factor | n | (+) | (-) | Positive (%) | P value |
| Sex | |||||
| M | 86 | 40 | 46 | 46.51 | |
| F | 16 | 3 | 13 | 18.75 | 0.039 |
| Age (yr) | |||||
| < 60 | 58 | 27 | 31 | 46.55 | |
| ≥ 60 | 44 | 16 | 28 | 36.36 | 0.302 |
| Histological grade | |||||
| Well | 32 | 7 | 25 | 21.88 | |
| Moderate & poor | 70 | 36 | 34 | 51.43 | 0.005 |
| Lymph node metastasis | |||||
| (-) | 55 | 24 | 31 | 43.64 | |
| (+) | 47 | 19 | 28 | 40.43 | 0.743 |
| Location1 | |||||
| Upper | 11 | 4 | 7 | 36.36 | |
| Middle | 55 | 22 | 33 | 40.00 | |
| Lower | 36 | 17 | 19 | 47.22 | 0.822 |
| T Stage | |||||
| T0-1 | 7 | 4 | 3 | 57.14 | |
| T2-4 | 95 | 39 | 56 | 41.04 | 0.405 |
| Factor | n | (+) | (-) | Positive (%) | P value |
| Sex | |||||
| M | 86 | 46 | 40 | 53.49 | |
| F | 16 | 9 | 7 | 56.25 | 0.839 |
| Age (yr) | |||||
| < 60 | 58 | 33 | 25 | 56.90 | |
| ≥ 60 | 44 | 22 | 22 | 50.00 | 0.489 |
| Histological grade | |||||
| Well | 32 | 21 | 11 | 65.63 | |
| Moderate | 50 | 27 | 23 | 54.00 | |
| Poor | 20 | 7 | 13 | 35.00 | 0.031 |
| Lymph node metastasis | |||||
| (-) | 55 | 31 | 24 | 56.36 | |
| (+) | 47 | 24 | 23 | 51.06 | 0.592 |
| Location1 | |||||
| Upper | 11 | 5 | 6 | 45.45 | |
| Middle | 55 | 29 | 26 | 52.73 | |
| Lower | 36 | 21 | 15 | 58.33 | 0.660 |
| T Stage | |||||
| T0-1 | 7 | 3 | 4 | 2.86 | |
| T2-4 | 95 | 52 | 43 | 54.74 | 0.543 |
Nearly 50% of patients with the diagnosis of esophageal cancer present with overt metastatic disease, and chemotherapy is the mainstay of palliation in this setting. With the increasing use of chemotherapy as an adjunct to surgical management, systemic chemotherapy will ultimately be used to treat the majority of patients with esophageal cancer. The combination of 5-fluorouracil (5-FU) and cisplatin is widely used in the treatment of esophageal cancer. Alternatively, taxane or irinotecan is applied in combination with either 5-FU or cisplatin[1]. The response to cisplatin and 5-FU has been low, ranging from 35% to 40%[2]. Therefore, there is great interest in identifying additional biochemical markers that might be predictive of chemotherapy response and resistance. As Ilson et al[1] stated: “future strategies in the treatment of esophageal carcinoma will undoubtedly be based on advances in the understanding of the molecular biology of the disease”.
Thymidylate synthase (TS) is a key enzyme for DNA and RNA synthesis. The anticancer activity of 5-FU is based on this molecular target. Glutathione-s-transferase π (GST-π) actively binds to platinum and allows it to be removed from the cytosol[3]. Several studies have suggested that the expression of TS and GST-π could be associated with chemotherapy resistance and prognosis in esophageal cancer and gastric cancer patients[3-6]. To our knowledge, the significance of TS and GST-π expression in esophageal squamous cell carcinoma (SCC) has not been reported to date in a Chinese population.
The current study was conducted (1) to investigate the expression characteristics of TS and GST-π in esophageal SCC (ESCC) in a Chinese population, and (2) to study the association between TS, GST-π and the clinical characteristics of the patients.
Immunohistochemical analysis has been used to assess the expression of molecular markers in malignant tumors. TS or GST-π has been shown to predict chemotherapy response and resistance in several cancers[7-12].
In this study, we determined the expression of TS and GST-π in surgically resected formalin-fixed, paraffin-embedded ESCC tissue sections from 102 patients and in 28 normal esophageal mucosa samples using immunohistochemical methods. The patients (86 males and 16 females) with ESCC underwent surgical resection at the Department of Thoracic Surgery, People’s Hospital of Taizhou (Taizhou Medical School, Yangzhou & Nantong University), between August 2005 and September 2007. All patients had undergone a subtotal or total esophagectomy and radical lymph node dissection.
Histopathological specimens were fixed in 10% buffered formalin, routinely processed, and embedded in paraffin. All specimens were obtained from patients who had not received chemo- or radiotherapy prior to surgical resection. All hematoxylin and eosin stained sections were reviewed and reexamined by pathologists. The grade of tumor differentiation was determined according to the classification of the World Health Organization[13], and staged according to the TNM classification[14].
The patients were 35-76 years of age with a median age of 58.0 years. The location of the tumors was as follows: upper intra-thoracic esophagus in 11 cases (10.7%); middle intra-thoracic esophagus in 55 cases (53.9%); lower intra-thoracic esophagus in 36 cases (35.2%). Histological degree of differentiation was well-differentiated in 32 cases (31.4%), moderately-differentiated in 50 cases (49.1%) and poorly-differentiated in 20 cases (19.6%). Five cases were Stage I, 47 cases were Stage II, 33 cases were Stage III and 17 cases were Stage IV. NEM samples were taken from 28 patients from an area more than 5 cm from the cancerous tissue, as control non-tumor samples.
The following antibodies were used in this study: mouse monoclonal antibody, anti-human TS and GST-π, the PV-9000 test kit (Zhongshan Goldenbridge Biotechnology Co., LTD, Beijing, China) was also used.
The specimens with adjacent non-cancerous esophageal mucosa were cut into 4-5-μm thick sections and mounted onto slides, deparaffinized with xylene, and rehydrated with graded concentrations of ethanol. Endogenous peroxidase activity was blocked by incubating with 3% hydrogen peroxide (H2O2) in deionized water for 10 min. The slides were washed three times with TBS buffer (10 mmol/L Tris-HCl, 100 mmol/L NaCl, pH 7.5) for 2 min. Before application of the TS primary antibody, an antigen retrieval technique was used (10 mmmol/L sodium citrate solution, pH 6.0 in a rice cooker, at 640 W for 30 min). After three washes with TBS, an aliquot of 100 μL of primary antibody was then applied to each section and incubated at 4°C overnight. It is not necessary to perform an antigen retrieval technique for GST-π. After washing 3 times with TBS and following the directions in the kit manual, agent one and then agent two (including the kit) were applied for 20 min at RT. Finally, the sections were washed 3 times with TBS, and the immunoreactions were visualized with 0.0067% diaminobenzidine as the substrate with 0.03% H2O2 in 100 mmmol/L Tris-HCl buffer for 3 min. The sections were lightly counterstained in Haris hematoxylin solution for microscopic examination. Simultaneously, each section was incubated with TBS instead of the primary antibody as an internal negative control.
The immunostained specimens were analysed by two independent pathologists. Cytoplasm and or nuclear staining (brown reaction product) was regarded as a positive result. Five fields in each tumor and non-tumor section were evaluated at medium power (× 200) to determine the proportion of tumor cells and the staining intensity of the cytoplasm and or nuclei in each section. The percentage of positive tumor cells was assigned to one of the following categories: 0 (0%-4%), 1 (5%-24%), 2 (25%-49%), 3 (50%-74%), or 4 (75%-100%). The intensity of immunostaining was determined as 0 (negative), 1+ (weak), and 2+ (strong). Additionally, an immunoreactive score was calculated by multiplying the percentage of positive cells and the staining intensity. The average score in each tumor and non-tumor section was calculated, and the expression was considered positive when the score was > 2[15]. To confirm the reproducibility of the results, all sections were scored twice, the highest score between the 2 observers are thus reported.
The correlations between the expression of TS, GST-π and clinicopathological factors were determined using the χ2 or Fisher test (SPSS 15.0 software package) at the 5% level.
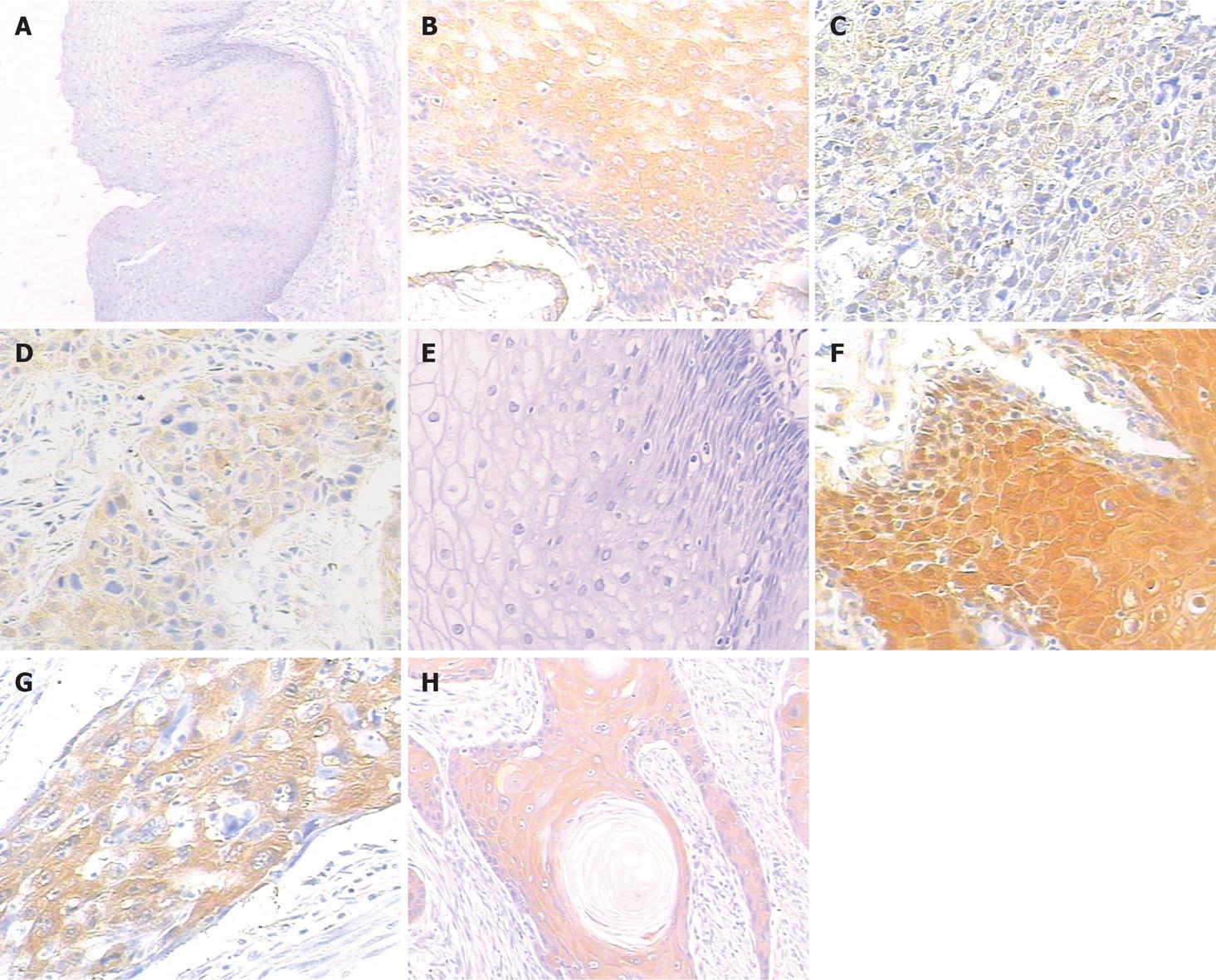
Without specific primary antibody to TS, no staining was observed in esophageal specimens (Figure 1A). The staining of TS was mainly concentrated in the cytoplasm of cells, occasionally, the nuclei were also stained (Figure 1B). The expression rates of TS in normal esophageal mucosa and in ESCC were 17.86% (5/28) and 42.16% (43/102), respectively. This suggested that the expression level of TS in ESCC was significantly higher than that in normal esophageal mucosa (χ2 = 5.50, P = 0.018).
The correlations between the expression of TS and the clinicopathologic features of ESCC are summarized in Table 1. The expression of TS was not significantly associated with age of the patients, tumor size, lymph node metastasis, depth of invasion or tumor stage. The expression of TS was significantly higher in poorly- and moderately-differentiated ESCC (Figure 1C) than in well-differentiated ESCC (Figure 1D) (χ2 = 7.866, P < 0.01). Female patients had tumors with low TS expression (18.75%) more frequently than male patients (46.51%) (χ2 = 4.264, P < 0.05).
Without specific primary antibody to GST-π, no staining was observed in esophageal specimens (Figure 1E). While the staining of GST-π was mainly concentrated in the cytoplasm, occasionally, the nuclei of cells were also stained (Figure 1F). The positive expression rates of GST-π in normal esophageal mucosa and in ESCC were 78.57% (22/28) and 53.92% (55/102), respectively. This showed that the expression level of GST-π in normal esophageal mucosa was significantly higher than that in ESCC (χ2 = 5.528, P < 0.05).
The correlations between the positive expression rates of GST-π and the clinicopathologic features of ESCC are summarized in Table 2. The positive expression rates of GST-π were not significantly associated with the sex or age of the patients, tumor size, lymph node metastasis, depth of invasion or tumor stage. However, positive expression was significantly higher in well-differentiated ESCC (Figure 1H) than in poorly-differentiated ESCC (Figure 1G) (χ2 = 4.645, P < 0.05).
The present study was designed to evaluate the expression characteristics of TS and GST-π in ESCC, and to assess the relationship between TS, GST-π and clinical characteristics. Chemotherapy with cisplatin/5-FU is accepted as a standard treatment in squamous cell and adenocarcinoma of the esophagus. TS is the enzyme targeted by 5-FU, and this may be a potential marker of chemotherapy response, whereas an increase in expression of TS may indicate resistance to 5-FU[16]. Assessment of the probability of chemotherapy resistance using immunohistochemistry methods detecting TS and GST-π expression may allow for the selection of a more effective chemotherapeutic regimen in several cancer patients[8-12].
TS plays an important role in folate metabolism. Using the methyltetrahydrofolic acid as a substrate, TS catalyses the methylation of deoxyuridylic acid, transferring it into deoxythymidylic acid, which is an important nucleotide in the synthesis and reparation of DNA[17]. This study showed that the expression of TS in ESCC was higher than that in normal tissue, and that the expression in moderately- and poorly-differentiated ESCC was higher than that in well-differentiated ESCC. This study also revealed that there is a potential to select patients according to whether TS expression is correlated with chemosensitivity to 5-FU. Some studies have indicated that esophageal cancer in patients with low expression of TS is more sensitive to chemotherapy than those with high expression[3,4].
In the current study, the expression level of TS was observed to be associated with gender. The expression level of TS was significantly higher in males than in females, contrary to the findings reported by Dong[4]. More studies are needed to investigate the significance of this difference, if any, to the outcome of patients.
In our study, the proportion of male to female patients was 5.4 to 1, this was similar to that of Joshi (6.07 to 1)[3]. If we can verify that the expression of TS is higher in male than female patients, then this result may help us to understand why esophageal squamous cell carcinoma is related to gender.
GST is a group of isozymes with the function of detoxification and combining proteins. In humans, GST contains α, μ, π, σ and θ, 5 family constellations and 13 different enzymes. The constellations are encoded by GSTA, GSTM, GSTP, GSTS, GSTT, respectively, and the relationship between GST-π and tumors has attracted much attention[18]. GST-π not only affects cisplatin by shifting it away from the cells, but it can also release oxygen free radicals, a mechanism which reduces radiation damage[2]. The expression of GST-π was higher in normal esophageal mucosa than in ESCC, and was higher in well-differentiated tumors compared to poorly-differentiated tumors. These results suggest that the loss of GST-π expression in esophageal epithelium may be an early pre-cancerous sign. The expression of GST-π had no significant association with gender, age, location of the tumor, lymph node metastasis or T stage, consistent with a previous report[19]. Recently, a study in gastric cancer indicated that the expression of GST-π may be associated with the efficacy of cisplatin[6].
In conclusion, the present results indicate that expression of TS and GST-π in ESCC in a Chinese population may add to understanding tumor characteristics and to predict response to chemotherapy. It is possible to predict chemotherapy response and resistance by detecting these biological markers. Thus, we are planning to investigate the relationship between expression of TS, GST-π, the curative effect of chemotherapy, and survival rate in patients with ESCC in a future study.
Esophageal cancer is a serious threat to human health, and nearly 50% of patients with a diagnosis of esophageal cancer present with overt metastatic disease. Chemotherapy has played a crucial role in the treatment of esophageal cancer. The combination of 5-fluorouracil and cisplatin is widely used in these patients. The response to these two drugs has been low. Therefore, it is important to know which drugs may induce a response in these patients.
Thymidylate synthase (TS) is a key enzyme for DNA and RNA synthesis. The anticancer activity of 5-FU is based on this molecular target. Glutathione-s-transferase π (GST-π) actively binds to platinum and allows it to be removed from the cytosol. In this study, we demonstrated that the expression of TS and GST-π was related to clinicopathological factors of esophageal cancer.
Recently, a number of studies have suggested that the expression of TS and GST-π is associated with chemotherapy resistance and prognosis in esophageal cancer and gastric cancer patients. However, the study of TS and GST-π in esophageal squamous cell carcinoma (ESCC) has not been reported in a Chinese population. Their study showed that the expression features of TS and GST-π were obviously different in normal esophageal mucosa and ESCC. Furthermore, the expression level of TS and GST-π is related to clinicopathological factors of ESCC in a Chinese population.
By understanding the anti-cancer molecular target of 5-FU and platinum and their relationships with TS and GST-π, this study indicated that it may be possible to predict response and resistance to chemotherapy by detecting TS and GST-π in patients with ESCC.
The expression level of TS in ESCC was higher than in normal esophageal mucosa. In contrast, GST-π in normal esophageal mucosa was higher than that in ESCC. These enzymes can be used as diagnostic molecular markers for ESCC. The results demonstrated that tumor tissues were poorly differentiated when the expression of TS was increased and the expression of GST-π was reduced.
This is well written article, describing the correlation between expression of TS and GST-π with clinicopathological features in patients with ESCC.
Peer reviewer: You-Yong Lu, Professor, Beijing Molecular Oncology Laboratory, Peking University School of Oncology and Beijing Institute for Cancer Research, #52, Fucheng Road, Haidian District, Beijing 100036, China
S- Editor Li LF L- Editor Webster JR E- Editor Yin DH
| 1. | Ilson DH. Esophageal cancer chemotherapy: recent advances. Gastrointest Cancer Res. 2008;2:85-92. [Cited in This Article: ] |
| 2. | Bleiberg H, Conroy T, Paillot B, Lacave AJ, Blijham G, Jacob JH, Bedenne L, Namer M, De Besi P, Gay F. Randomised phase II study of cisplatin and 5-fluorouracil (5-FU) versus cisplatin alone in advanced squamous cell oesophageal cancer. Eur J Cancer. 1997;33:1216-1220. [Cited in This Article: ] |
| 3. | Joshi MB, Shirota Y, Danenberg KD, Conlon DH, Salonga DS, Herndon JE 2nd, Danenberg PV, Harpole DH Jr. High gene expression of TS1, GSTP1, and ERCC1 are risk factors for survival in patients treated with trimodality therapy for esophageal cancer. Clin Cancer Res. 2005;11:2215-2221. [Cited in This Article: ] |
| 4. | Dong ZM, Cui YJ, Kuang G, Wang R, Yu FL, Zhang JH. [Polymorphisms of thymidylate synthase gene and correlation of its protein expression to lymph node metastasis of esophageal squamous cell carcinoma]. Ai Zheng. 2005;24:1225-1229. [Cited in This Article: ] |
| 5. | Kuramochi H, Tanaka K, Oh D, Lehman BJ, Dunst CM, Yang DY, De Meester SR, Hagen JA, Danenberg KD, De Meester TR. Thymidylate synthase polymorphisms and mRNA expression are independent chemotherapy predictive markers in esophageal adenocarcinoma patients. Int J Oncol. 2008;32:201-208. [Cited in This Article: ] |
| 6. | Goekkurt E, Hoehn S, Wolschke C, Wittmer C, Stueber C, Hossfeld DK, Stoehlmacher J. Polymorphisms of glutathione S-transferases (GST) and thymidylate synthase (TS)--novel predictors for response and survival in gastric cancer patients. Br J Cancer. 2006;94:281-286. [Cited in This Article: ] |
| 7. | Huang JX, Yan W, Song ZX, Qian RY, Chen P, Salminen E, Toppari J. Relationship between proliferative activity of cancer cells and clinicopathological factors in patients with esophageal squamous cell carcinoma. World J Gastroenterol. 2005;11:2956-2959. [Cited in This Article: ] |
| 8. | Kim SH, Kwon HC, Oh SY, Lee DM, Lee S, Lee JH, Roh MS, Kim DC, Park KJ, Choi HJ. Prognostic value of ERCC1, thymidylate synthase, and glutathione S-transferase pi for 5-FU/oxaliplatin chemotherapy in advanced colorectal cancer. Am J Clin Oncol. 2009;32:38-43. [Cited in This Article: ] |
| 9. | Yasumatsu R, Nakashima T, Uryu H, Ayada T, Wakasaki T, Kogo R, Masuda M, Fukushima M, Komune S. Correlations between thymidylate synthase expression and chemosensitivity to 5-fluorouracil, cell proliferation and clinical outcome in head and neck squamous cell carcinoma. Chemotherapy. 2009;55:36-41. [Cited in This Article: ] |
| 10. | Ohrling K, Edler D, Hallström M, Ragnhammar P. Expression of thymidylate synthase in liver and lung metastases of colorectal cancer and their matched primary tumours. Anticancer Res. 2008;28:1741-1747. [Cited in This Article: ] |
| 11. | Miyoshi T, Kondo K, Toba H, Yoshida M, Fujino H, Kenzaki K, Sakiyama S, Takehisa M, Tangoku A. Predictive value of thymidylate synthase and dihydropyrimidine dehydrogenase expression in tumor tissue, regarding the efficacy of postoperatively administered UFT (tegafur+uracil) in patients with non-small cell lung cancer. Anticancer Res. 2007;27:2641-2648. [Cited in This Article: ] |
| 12. | Kwon HC, Roh MS, Oh SY, Kim SH, Kim MC, Kim JS, Kim HJ. Prognostic value of expression of ERCC1, thymidylate synthase, and glutathione S-transferase P1 for 5-fluorouracil/oxaliplatin chemotherapy in advanced gastric cancer. Ann Oncol. 2007;18:504-509. [Cited in This Article: ] |
| 13. | Watanabe H, Jass JR, Sobin LH. World Health Organization. International histological classification of the tumors: histological typing of esophageal and gastric tumors. 2nd ed. Berlin: Springer-Verlag 1992; . [Cited in This Article: ] |
| 14. | Sobin LH, Wittekind CH. UICC TNM classification of malignant tumors. 5th edition. New York: John Wiley Sons. Inc 1997; . [Cited in This Article: ] |
| 15. | Kawasaki H, Altieri DC, Lu CD, Toyoda M, Tenjo T, Tanigawa N. Inhibition of apoptosis by survivin predicts shorter survival rates in colorectal cancer. Cancer Res. 1998;58:5071-5074. [Cited in This Article: ] |
| 16. | Lenz HJ, Leichman CG, Danenberg KD, Danenberg PV, Groshen S, Cohen H, Laine L, Crookes P, Silberman H, Baranda J. Thymidylate synthase mRNA level in adenocarcinoma of the stomach: a predictor for primary tumor response and overall survival. J Clin Oncol. 1996;14:176-182. [Cited in This Article: ] |
| 17. | Wang LD, Guo RF, Fan ZM, He X, Gao SS, Guo HQ, Matsuo K, Yin LM, Li JL. Association of methylenetetrahydrofolate reductase and thymidylate synthase promoter polymorphisms with genetic susceptibility to esophageal and cardia cancer in a Chinese high-risk population. Dis Esophagus. 2005;18:177-184. [Cited in This Article: ] |
| 18. | Lee JM, Wu MT, Lee YC, Yang SY, Chen JS, Hsu HH, Huang PM, Kuo SW, Lee CJ, Chen CJ. Association of GSTP1 polymorphism and survival for esophageal cancer. Clin Cancer Res. 2005;11:4749-4753. [Cited in This Article: ] |
| 19. | Li Z, Zhang R, Luo X. [Expression of glutathione S-transferase-pi in human esophageal squamous cell carcinoma]. Zhonghua Zhongliu Zazhi. 2001;23:39-42. [Cited in This Article: ] |