Published online Sep 7, 2009. doi: 10.3748/wjg.15.4156
Revised: July 28, 2009
Accepted: August 4, 2009
Published online: September 7, 2009
AIM: To determine the effect of chemotherapy on wound healing by giving early preoperative 5-fluorouracil (5-FU) to rats with colonic anastomoses.
METHODS: Sixty Albino-Wistar male rats (median weight, 235 g) were used in this study. The rats were fed with standard laboratory food and given tap water ad libitum. The animals were divided into three groups: Group 1: Control group (chemotherapy was not administered), Group 2: Intraperitoneally (IP) administered 5-FU group (chemotherapy was administered IP to animals at a dose of 20 mg/kg daily during the 5 d preceeding surgery), Group 3: Intravenously (IV) administered 5-FU group. Chemotherapy was administered via the penil vein, using the same dosing scheme and duration as the second group. After a 3-d rest to minimize the side effects of chemotherapy, both groups underwent surgery. One centimeter of colon was resected 2 cm proximally from the peritoneal reflection, then sutured intermittently and subsequently end-to-end anastomosed. In each group, half the animals were given anaesthesia on the 3rd postoperative (PO) day and the other half on the 7th PO day, for in vivo analytic procedures. The abdominal incisions in the rats were dissected, all the new and old anastomotic segments were clearly seen and bursting pressures of each anastomotic segment, tissue hydroxyproline levels and DNA content were determined to assess the histologic tissue repair process.
RESULTS: When the IV group was compared with the IP group, bursting pressures of the anastomotic segments on the 3rd and 7th PO days, were found to be significantly decreased, hydroxyproline levels at the anastomotic segment on the 7th PO day were significantly decreased (P < 0.01).
CONCLUSION: In this study, we conclude that early preoperative 5-FU, administered IV, negatively affects wound healing. However, IP administered 5-FU does not negatively affect wound healing.
- Citation: Ozel L, Ozel MS, Toros AB, Kara M, Ozkan KS, Tellioglu G, Krand O, Koyuturk M, Berber I. Effect of early preoperative 5-fluorouracil on the integrity of colonic anastomoses in rats. World J Gastroenterol 2009; 15(33): 4156-4162
- URL: https://www.wjgnet.com/1007-9327/full/v15/i33/4156.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4156
| Groups | Leukocytes (109/L) | Platelets (109/L) | Haemoglobin (g/dL) | |||
| 3rd d | 7th d | 3rd d | 7th d | 3rd d | 7th d | |
| Control | 9.0 ± 3.04 | 7.6 ± 3.4 | 1117 ± 169 | 1270 ± 229 | 11 ± 1.7 | 12.6 ± 1.2 |
| IP | 9.1 ± 3.3 | 9.7 ± 2.4 | 808 ± 203 | 1228 ± 194 | 10.5 ± 1.7 | 11.9 ± 0.45 |
| IV | 7.4 ± 4.7 | 15.9 ± 5.2 | 736 ± 243 | 1542 ± 289 | 9.6 ± 1.13 | 11.16 ± 1.9 |
| Groups | 3rd d | 7th d | ||||
| Control | IP | IV | Control | IP | IV | |
| Granulation | 1.66 ± 0.70 | 2.14 ± 0.89 | 1.42 ± 0.53 | 2.66 ± 0.50 | 2.71 ± 0.48 | 2.57 ± 0.53 |
| Re-epithelization | 1.55 ± 1.66 | 2.71 ± 2.21 | 1.00 ± 0.00 | 4.77 ± 1.20 | 5.28 ± 0.95 | 2.42 ± 2.14 |
| Muscular tissue | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.22 ± 0.44 | 1.42 ± 0.44 | 1.14 ± 0.37 |
Colorectal cancer is a common malignancy in most developed countries worldwide[1]. Metastasis frequently occurs before clinical detection of the primary tumour. Despite the advances in surgical techniques, this characteristic of the malignancy prevents a significant improvement in cure rates for colorectal cancers[2]. While cancer therapy was limited to surgery in the past, nowadays therapy includes the combination of surgical radiotherapy, chemotherapy, hormonal and biological therapies[3,4]. 5-fluorouracil (5-FU) treatment has been accepted as standard chemotherapy for colorectal cancers for a long time. Recently, high recurrence rates, the presence of distant metastasis, the possibility of complete resection, and the removal of circulating tumour cells after curative resection of colorectal cancers constitute a new therapeutic approach known as neo-adjuvant chemotherapy[5-7]. While the integrity of anastomoses after colorectal cancer resection is an important parameter on mortality and morbidity, the effects of preoperative chemotherapy on wound healing and anastomoses are also important and have not been clearly outlined.
This study investigates the effects of early preoperative administration of 5-FU, given intravenously (IV) and intraperitoneally (IP), on wound healing in colon anastomoses.
Sixty male Wistar-Albino rats weighing between 225 and 315 g were used in this study. All rats were clinically healthy and were fed with standard laboratory food and water. The animals were numbered at the beginning of the study and weighed every day during the study. There were three groups: Group I, a control group (n = 20); group II which received 5-FU IP (n = 20); group III which received 5-FU IV (n = 20). The study was approved by the local Ethics Committee of Haydarpaşa Numune Hospital.
When pilot studies and related literature were taken into account, a dose of 20 mg/kg 5-FU was calculated to be the maximum non-lethal dose[8]. Chemotherapy was not administered to the control group. The second group was administered 5-FU IP at a dose of 20 mg/kg in saline at a concentration of 5 mg/mL each day prior to surgery. The third group was given 5-FU via the penile vein at a dose of 20 mg/kg in saline at a concentration of 2 mg/mL each day for 5 d before surgery. Both groups underwent surgery on the 3rd d after chemotherapy to reduce the adverse effects of chemotherapy.
The same operative procedure was performed in all groups by the same surgeon. 10 mg/kg of ketamine was given subcutaneously to rats under ether anaesthesia. After shaving the frontal abdominal wall, this area was cleaned with povidone iodine and covered with sterile cloths. The abdomen was entered through a 3 cm mid-line incision, 1 cm of colon 2 cm proximal of the peritoneal reflection was resected, and a side-to-side anastomosis was made using ten intermittent sutures with 6/0 polypropylene (Ethicon). Muscles of the front abdominal wall and skin were closed by continuous suture with 3/0 silk. Half the animals in each group were anaesthetized again either on day 3 or 7 after surgery for in vivo analytic procedures. The animals were then killed by haemorrhage for in vitro analytic procedures.
After making an abdominal incision, macroscopic evaluations of the anastomotic segment were performed. Adhesions surrounding the anastomoses were not cut, and bursting pressure was measured for every anastomotic segment during the internal passage of 200 mL/h saline. For this purpose, a 10/0 silicon catheter was passed via the anus to 2 cm distal of the anastomosis and the colon was ligated with silk suture above the catheter. The colon was cut 3 cm proximal to the anastomotic segment. The catheter, which had its end fixed to a standard sphygmomanometer (Petaş, Turkey), was moved 1 cm to the anastomotic segment from the cut end of the colon and the colon was ligated with silk suture around the catheter. The perfusator (Becton Dickinson, France) was maintained at a speed of 200 mL/h and saline was given continuously through the catheter situated in the anus. Increased pressure on the sphygmomanometer was observed. Pressure values of the first leakage from the anastomotic segments, when increased pressure on the sphygmomanometer stopped and the time of falling pressure were recorded as bursting pressures. These bursting pressures were recorded in mmHg for each animal.
After measuring the anastomotic pressures and just before the animals were sacrificed, 5 mL blood samples were taken from the inferior vena cava to determine white blood cell, haemoglobin and platelet counts.
Anastomotic segments were isolated from the surrounding tissues. One cm of colonic segment, including the anastomotic area was resected and was longitudinally separated into two parts. One of the segments was frozen at -45°C for hydroxyproline measurements (hydroxyproline was used as a marker of collagen content) which were calculated as nanograms per gram of tissue. Anastomotic hydroxyproline content was measured by spectrophotometric determination using the method described by Bergman et al[9]. The other segment was embedded in paraffin for DNA content measurement and histological assay. Tissue DNA content was determined by flow cytometry using Mod-Fit Ver 5.01 software[10].
Tissue sections from routinely embedded paraffin blocks were stained with hematoxylin-eosin and examined by light microscopy. Histological examination was evaluated using the criteria determined by de Roy van Zuidewijn et al[11]. Slides were evaluated twice by the same observer in a blind fashion. Granulation tissue was evaluated as; 1-low; 2-medium; 3-high (intense) and histological parameter scores related to muscular tissue were evaluated as; 1-negative; 2-medium; 3-complete. According to a seven-point scale, mucosal re-epithelization scores were as follows; 1-negative, 2-little-one line cubic, 3-lot-one line cubic, 4-nearly one line cubic, 5-finished-one line cubic, 6-one line glandular, 7-normal glandular mucosa.
All data are presented as means ± SE. Non-parametric Kruskall Wallis variant analysis was used for multiple group comparisons of statistical analysis and subgroup comparisons were performed with Dunn’s test. Pair group analysis was evaluated using Mann-Whitney U test.
During the experimental procedure, two animals died on the 3rd and 7th postoperative (PO) days in the IV group. The cause of death was leakage from the anastomotic site. No mortality was observed in the control and IP groups. Dead rats were replaced with new animals.
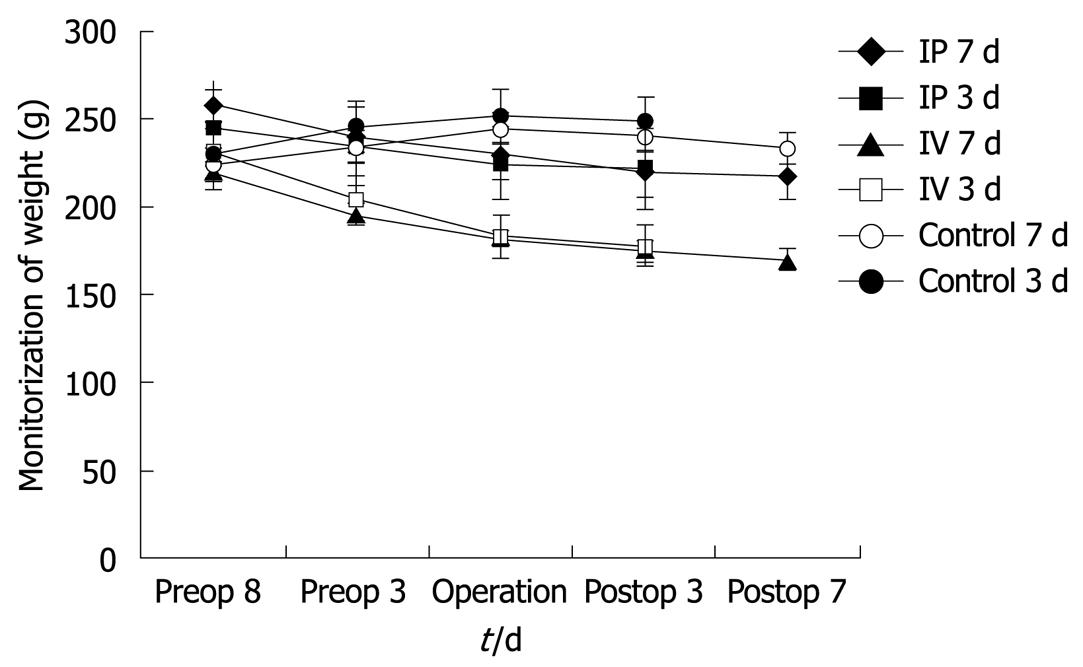
Rats were weighed at the beginning of the study, during the chemotherapy period and in the postoperative period. Weight loss was observed in all animals in the chemotherapy groups (IP and IV) compared with baseline values, whereas control animals gained weight during the preoperative period. Animals in the control group also lost weight during the PO period. Weight loss in the rats is shown in Figure 1. On the 3rd PO day, the IV 5-FU group weighed significantly less than the control group (P < 0.01) and the IP group (P < 0.01). Weight loss in the IV 5-FU group on the 7th PO day was found to be significantly lower than the control group (P < 0.01) and the IP group (P < 0.001).
Haemoglobin, white blood cell and platelet counts in the blood samples taken on the 3rd and 7th PO days after chemotherapy are shown in Table 1. There was no statistically significant difference between the groups for haemoglobin counts on the 3rd and 7th PO days. On the 3rd PO day in the IV 5-FU group, the platelet count was found to be significantly lower than the control group (P < 0.05).
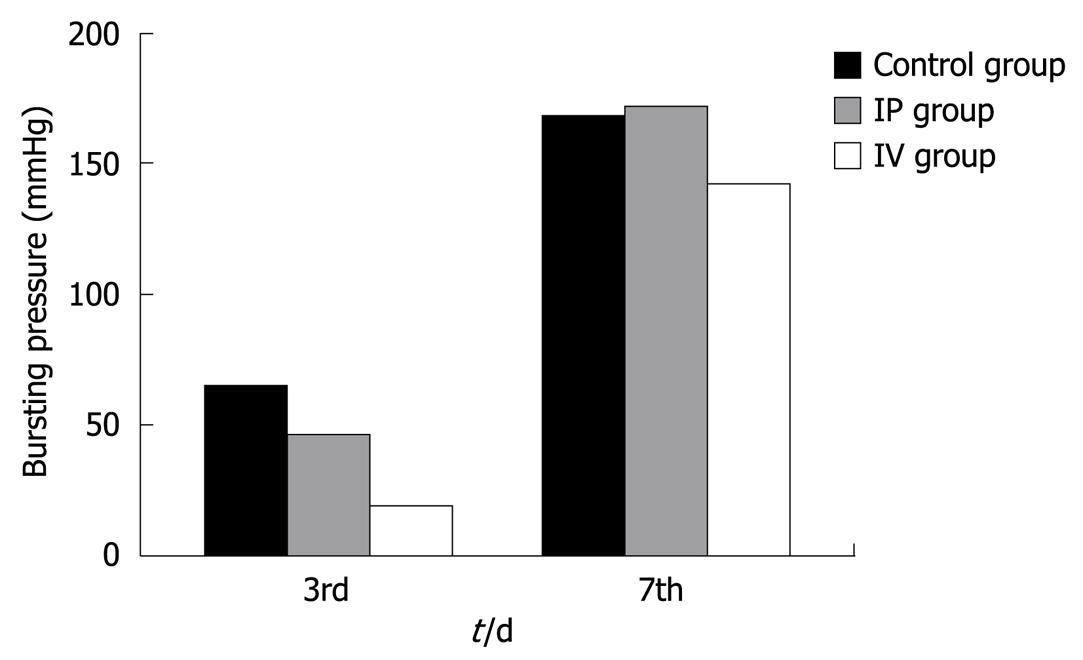
Bursting pressures were measured at anastomotic segments on the 3rd and 7th PO days (Figure 2). Bursting pressure values were significantly lower than the control (P < 0.01) and IP group on (P < 0.05) the 3rd PO day in the IV 5-FU group. On the 7th PO day, bursting pressure values in the IV and IP groups were not significantly different from the control group. However, bursting pressure values in the IV 5-FU group were significantly lower when compared with the IP group (P < 0.01).
Anastomotic hydroxyproline values were statistically significantly higher in the IP group compared to the control and IV group on the 3rd PO day (P < 0.01). There were no statistically significant differences between the IV and control groups on PO day 3, regarding hydroxyproline levels. Hydroxyproline levels in the IP group were the highest and anastomotic hydroxyproline levels in the IV group were significantly lower than the IP group on PO day 7 (P < 0.01) (Figure 3).
DNA contents in the anastomotic segments were evaluated using a flow cytometer technique in this study[10]. When the anastomotic segments were examined, a decrease in cell proliferation was noted on the 3rd PO day in the IV group, when compared to the control group, and on the 7th PO day between the control and the IP group. However, this decrease was not statistically significant. The tissue proliferation index of anastomotic segments is shown in Figure 4.
Scores for the histological parameters in the groups are given in Table 2. The best granulation tissue was observed on the 3rd and 7th d in the IP 5-FU group. On the 3rd d of re-epithelization, there was no statistically significant difference between the control and both 5-FU administered groups. However, on the 7th d there was a statistically significant difference between the control and the IV 5-FU group (P < 0.05), and between the IP 5-FU and IV 5-FU groups (P < 0.05). On multiple comparisons between the groups, the statistically significant difference was maintained (P < 0.05). Muscle tissue formation was not complete on the 3rd and 7th d and related values were not statistically different between the groups.
Colorectal cancer is the second most frequent type of cancer in industrialized countries. Despite improvements in surgical techniques, almost half of patients with colorectal cancer will eventually die of recurrent disease.
When colon cancer recurs after surgical resection, local disease is found in 25%-40% of patients, peritoneal implants in 12%-28% of patients, and liver metastases in 40% of patients. Hepatic and peritoneal metastases have been reported to be the most frequent failure patterns in resected colorectal cancer patients[12,13].
Solomon et al[14] reported on the incidence of colorectal cancer cells on peritoneal surfaces. Overall, 15% of patients had positive cytology for cancer cells in the peritoneal or bowel surface. Stage II and III disease is treated with wide surgical resection in combination with adjuvant or neo-adjuvant chemoradiation. The combined modality of chemotherapy and surgery increases overall survival and the disease-free period[15-18]. 5-FU was first reported to be effective for colorectal cancer in the 1950s. For more than 10 years, 5-FU was the only adjuvant drug given as a single agent. Since its introduction, 5-FU has remained the cornerstone of adjuvant treatment for colorectal cancer. A number of clinical and experimental data are available on the effects of 5-FU, either alone or in combination with other chemotherapeutic agents, on wound healing[19-22].
In the adjuvant therapy of colorectal cancers, 5-FU was used alone in prolonged systemic regimens or in combination with other agents[22-25]. Fundamentals of preoperative chemotherapy were based on the results of Cole et al[5]. According to these results, numerous malignant cells pass into the peripheral circulation during surgical manipulations for localized carcinomas. Preoperatively administered cytotoxic agents may allow tumour resectability and decrease the incidence of distant or local metastases[26].
5-FU can be injected IV and hepatic concentrations can also be achieved intraportally and IP[22,27,28]. In addition, intravenous administration may not allow sufficient penetration in the abdominal cavity. This could be the reason why chemotherapeutic agents may not be effective enough to eliminate micrometastases, especially at the resection site and peritoneal surfaces, which are high risk sites for local recurrence. When the drug is administered via the intraperitoneal route, high local and hepatic concentrations can be achieved[28]. Local peritoneal recurrence and haematological toxicity were lower when 5-FU was administered IP. A prospective trial showed that 5-FU administered IP reduced peritoneal failure significantly more than intravenous 5-FU[29]. There is an increased interest in the use of intraperitoneal chemotherapy with 5-FU to treat advanced colon cancer. Randomised clinical trials have reported a reduction in local recurrence rate with either intraperitoneal 5-FU alone or combined with intravenous 5-FU[30].
Toxic side effects are the major dose-limiting factors in chemotherapy. In patients with advanced primary colon cancer, a significantly higher dose of 5-FU can be given by the intraperitoneal route than by the intravenous route[29]. We used the intraperitoneal approach to deliver high concentrations of 5-FU into the peritoneal cavity without increasing the risk of systemic toxicity. In addition, previous clinical and experimental studies have shown that immediate postoperative 5-FU given IP has no adverse effect on outcome[8].
In 1994, Kelsen et al[31] reported a phase I trial of postoperative intraperitoneal floxuridine and leucovorin plus systemic 5-FU and levamisole after resection of high-risk colon cancer. Intraperitoneal therapy appeared to be well tolerated, with no substantial increase in postoperative morbidity and no operative mortality.
In 1998, Scheithauer et al[30] accepted 241 patients with resected stage III or high-risk stage II colon cancer into a trial, comparing intravenous 5-FU and levamisole given for a period of 6 mo with a treatment program consisting of leucovorin plus 5-FU given IV and IP. In patients with stage III disease, a significant improvement in disease-free survival and overall survival rates was observed using the systemic plus intraperitoneal treatment, with an estimated 43% reduction in mortality rate. Intraperitoneal and systemic chemotherapy markedly reduced local regional recurrences[30].
It is stated that the highest non-lethal dose of 5-FU is 20 mg/kg for rats and this was the dose administered in our previous trial[11]. The 500 mg/m2 human dose is equal to the animal dose used in this study. A 3 d interval between the last day of chemotherapy and the surgical procedure was approved to reduce the strong side effects of chemotherapy[6,22].
Weight reductions were detected both during 5-FU injections and after surgery. The weight of the rats was recorded during the 5-FU injection period and days after the operation. All rats lost weight compared with baseline values during 5-FU administration and after the operation, which was statistically significant in the IV group. These results were concordant with reports from the literature[6,8,32]. Weight loss after 5-FU is related to anastomotic healing.
The burst pressure values of the anastomotic segments were measured in vivo on the 3rd and 7th PO days in rats receiving and not receiving 5-FU in this study, similar to that in the study by Kuzu et al[6]. Anastomotic burst pressures have been measured in vitro in most previous similar reports[8,22]. We think that in-vivo measurements reflect the clinical status much better. The bursting sites we found in this study are in accordance with those in other studies which reported that the anastomosis is the most common bursting point[22,33]. On the 3rd PO day, bursting pressure values for the IV 5-FU group were found to be significantly lower than the control and IP groups. On the 7th PO day, bursting pressure values in the IV 5-FU group were significantly lower than the IP group. Similar results have been found in previously reported studies[22,32,33].
Graf et al[32] reported that early postoperative 5-FU administration had a negative impact on the bursting strength of colonic anastomosis.
5-FU administered preoperatively may also have a negative influence on the ability of fibroblasts to proliferate and synthesize collagen. Reduced collagen synthesis can lead to anastomotic dehiscence[22].
Collagens which guarantee tissue continuity in the tissue repair period contain high proportions of glycine, proline and hydroxyproline. Tissue hydroxyproline levels are important parameters in the tissue repair process[34,35]. Some studies have found that 5-FU decreases the hydroxyproline content of wounds[19,21]. There were significant differences in hydroxyproline levels at the anastomotic segments on both the 3rd and 7th PO days in the IV group compared to the IP group. The decrease in collagen content was correspondant with the decrease in bursting pressures.
It is already known that the chemotherapeutic agent 5-FU inhibits DNA synthesis and cell proliferation, by affecting the cell cycle[36]. Based on this mechanism of action, the effect of chemotherapy on cell proliferation at the anastomotic segment was evaluated by DNA analysis. An obvious decrease in cell proliferation was found at the anastomotic segment of the IV chemotherapy group, compared with the IP and control groups on the 7th PO day. This decrease was not statistically significant. The effects of 5-FU on cancer kinetics determined by DNA analysis, was reported to stress the importance of neo-adjuvant chemotherapy. It was thought that available parameters could be used to determine the anti-tumour effects of 5-FU[37]. The DNA proliferation index can be used as a parameter to determine the effect of chemotherapy on tissue repair; however, further clinical and experimental studies are required.
We looked at histological aspects of the colon during a 7-d period. Although there were no statistically significant differences between the granulation tissues on the 3rd and 7th PO days in our study, the decrease in re-epithelization, as a sign of a mucosal improvement, was statistically significant in the IV group compared with the control and IP groups. According to the results of de Roy van Zuidewijn et al[11], completion of colonic muscular tissue repair takes about 21 d. Consistent with our study, muscular repairs were not complete in any of the groups at the 3rd and 7th d of the early tissue repair period, and no difference was found between the control and the experimental groups. Our histological findings suggested that IP 5-FU administration may be preferred over IV administration which was in accordance with our previous results.
All anastomoses were examined macroscopically during the second laparotomy, before the bursting pressure measurements. Intra-abdominal adhesions were not classified but the integrity of anastomoses, the existence of perianastomotic abscess or peritonitis and the formation of adhesions were less frequent in the IP group, when compared to the control and IV groups.
5-FU derivatives inhibit DNA synthesis by inhibiting thymidylate synthetase, and reduce the biological activity of RNA in both human cells and growing bacteria[38,39].
When applied locally, 5-FU seems to behave like an antibiotic. This confirms certain studies which implied an increasing bactericidal effect when antimicrobial drugs and antineoplastic drugs were administered together. Nyhlén et al[40] reported that against two of the three tested strains of Staphylococcus epidermidis. the combination of ciprofloxacin and 5-FU resulted in a synergistic prolongation of the postantibiotic effect (PAE) in comparison with the PAE induced by the drugs alone. However, these results need to be confirmed clinically.
In conclusion, our results demonstrate that, early preoperative IV administration of 5-FU has a negative effect on anastomotic healing of the colon. However, the IP route of 5-FU administration has no adverse effect on the healing process of colon anastomoses and burst pressure. This study was performed on healthy animals and may not reflect the exact situation in the case of malignant tumors.
For cancers of the large bowel, multi-institutional trials have demonstrated a significant reduction in mortality with adjuvant chemotherapy compared with surgery alone. 5-fluorouracil (5-FU) was therefore used as a neoadjuvant therapy in the present study where it had no adverse effect on anastomotic healing and burst pressure.
5-FU is the mainstay of systemic treatment for colorectal cancer. Intravenous administration may not allow sufficient penetration in the abdominal cavity. This may be the reason why chemotherapeutic agents are not effective enough to kill all micrometastases, especially at the resection site and peritoneal surfaces. Because of these theoretical advantages, neoadjuvant intraperitoneal chemotherapy may be a new treatment option for colorectal cancer. This study investigated the effect of preoperative 5-FU on the healing of colorectal anastomoses in the rat.
5-FU can be injected not only intravenously, but also intraportally and intraperitoneally (IP). When administered by the intraperitoneal route, high local and hepatic concentrations can be achieved. Whether 5-FU compromises wound healing is still controversial. Previous clinical and experimental studies have shown that immediate postoperative 5-FU given IP has no adverse effect on outcome. Therefore, 5-FU was used as neoadjuvant therapy in the present study.
This study may represent a future strategy for neoadjuvant chemotherapy in colonic cancer.
5-FU is the most widely used chemotherapeutic agent in the adjuvant treatment of colon cancer. 5-FU acts during synthesis by interfering with normal pathways. 5-FU might also have a negative influence on the ability of fibroblasts to proliferate and synthesize collagen. Reduced collagen synthesis could lead to anastomotic dehiscence.
It is an interesting topic for the readers of WJG. The authors described pre- and postoperative variables after two methods of 5-FU administration.
| 1. | Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106-130. [Cited in This Article: ] |
| 2. | Enblad P, Adami HO, Bergström R, Glimelius B, Krusemo U, Påhlman L. Improved survival of patients with cancers of the colon and rectum? J Natl Cancer Inst. 1988;80:586-591. [Cited in This Article: ] |
| 3. | Carethers JM. Review: Systemic treatment of advanced colorectal cancer: Tailoring therapy to the tumor. Therapeutic Advances in Gastroenterology. 2008;1:33-42. [Cited in This Article: ] |
| 4. | Balch CM, Pellis NR, Morton DL, Eifel PJ, Brennan MF. Oncology. Principles of Surgery. New York: McGraw Hill 1994; 305-376. [Cited in This Article: ] |
| 5. | Cole WH, Packard D, Southwick HW. Carcinoma of the colon with special reference to prevention of recurrence. J Am Med Assoc. 1954;155:1549-1553. [Cited in This Article: ] |
| 6. | Kuzu MA, Kuzu I, Köksoy C, Akyol FH, Uzal D, Kale IT, Orhan D, Terzi C. Histological evaluation of colonic anastomotic healing in the rat following preoperative 5-fluorouracil, fractionated irradiation, and combined treatment. Int J Colorectal Dis. 1998;13:235-240. [Cited in This Article: ] |
| 7. | Roberts S, Watne A, McGrath R, McGrew E, Cole WH. Technique and results of isolation of cancer cells from the circulating blood. AMA Arch Surg. 1958;76:334-346. [Cited in This Article: ] |
| 8. | de Waard JW, Wobbes T, Hendriks T. Early post-operative 5-fluorouracil does not affect the healing of experimental intestinal anastomoses. Int J Colorectal Dis. 1993;8:175-178. [Cited in This Article: ] |
| 9. | Bergman I, Loxley R. Two improved and simplified method for the spectrophotometric determination of hydroxyproline. Anal Chem. 1963;35:1961-1965. [Cited in This Article: ] |
| 10. | Baretton G, Gille J, Oevermann E, Löhrs U. Flow-cytometric analysis of the DNA-content in paraffin-embedded tissue from colorectal carcinomas and its prognostic significance. Virchows Arch B Cell Pathol Incl Mol Pathol. 1991;60:123-131. [Cited in This Article: ] |
| 11. | de Roy van Zuidewijn DB, Schillings PH, Wobbes T, de Boer HH. Histologic evaluation of wound healing in experimental intestinal anastomoses: effects of antineoplastic agents. Int J Exp Pathol. 1992;73:465-484. [Cited in This Article: ] |
| 12. | Minsky BD, Mies C, Rich TA, Recht A, Chaffey JT. Potentially curative surgery of colon cancer: patterns of failure and survival. J Clin Oncol. 1988;6:106-118. [Cited in This Article: ] |
| 13. | Gunderson LL, Sosin H. Areas of failure found at reoperation (second or symptomatic look) following "curative surgery" for adenocarcinoma of the rectum. Clinicopathologic correlation and implications for adjuvant therapy. Cancer. 1974;34:1278-1292. [Cited in This Article: ] |
| 14. | Solomon MJ, Egan M, Roberts RA, Philips J, Russell P. Incidence of free colorectal cancer cells on the peritoneal surface. Dis Colon Rectum. 1997;40:1294-1298. [Cited in This Article: ] |
| 15. | August DA, Ottow RT, Sugarbaker PH. Clinical perspective of human colorectal cancer metastasis. Cancer Metastasis Rev. 1984;3:303-324. [Cited in This Article: ] |
| 16. | Kehoe J, Khatri VP. Staging and prognosis of colon cancer. Surg Oncol Clin N Am. 2006;15:129-146. [Cited in This Article: ] |
| 17. | Levitan N. Chemotherapy in colorectal carcinoma. Surg Clin North Am. 1993;73:183-198. [Cited in This Article: ] |
| 18. | Ersoy E, Akbulut H, Moray G. Effects of oxaliplatin and 5-fluorouracil on the healing of colon anastomoses. Surg Today. 2009;39:38-43. [Cited in This Article: ] |
| 19. | van der Kolk BM, de Man BM, Wobbes T, Hendriks T. Is early post-operative treatment with 5-fluorouracil possible without affecting anastomotic strength in the intestine? Br J Cancer. 1999;79:545-550. [Cited in This Article: ] |
| 20. | Cunliffe WJ, Sugarbaker PH. Gastrointestinal malignancy: rationale for adjuvant therapy using early postoperative intraperitoneal chemotherapy. Br J Surg. 1989;76:1082-1090. [Cited in This Article: ] |
| 21. | Kuzu MA, Köksoy C, Kale T, Demirpençe E, Renda N. Experimental study of the effect of preoperative 5-fluorouracil on the integrity of colonic anastomoses. Br J Surg. 1998;85:236-239. [Cited in This Article: ] |
| 22. | Weiber S, Graf W, Glimelius B, Jiborn H, Påhlman L, Zederfeldt B. Experimental colonic healing in relation to timing of 5-fluorouracil therapy. Br J Surg. 1994;81:1677-1680. [Cited in This Article: ] |
| 23. | Laurie JA, Moertel CG, Fleming TR, Wieand HS, Leigh JE, Rubin J, McCormack GW, Gerstner JB, Krook JE, Malliard J. Surgical adjuvant therapy of large-bowel carcinoma: an evaluation of levamisole and the combination of levamisole and fluorouracil. The North Central Cancer Treatment Group and the Mayo Clinic. J Clin Oncol. 1989;7:1447-1456. [Cited in This Article: ] |
| 24. | Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Goodman PJ, Ungerleider JS, Emerson WA, Tormey DC, Glick JH. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med. 1990;322:352-358. [Cited in This Article: ] |
| 25. | Macdonald JS. Adjuvant therapy for colon cancer. CA Cancer J Clin. 1997;47:243-256. [Cited in This Article: ] |
| 26. | Diaz-Canton EA, Pazdur R. Adjuvant medical therapy for colorectal cancer. Surg Clin North Am. 1997;77:211-228. [Cited in This Article: ] |
| 27. | Brodsky JT, Cohen AM. Peritoneal seeding following potentially curative resection of colonic carcinoma: implications for adjuvant therapy. Dis Colon Rectum. 1991;34:723-727. [Cited in This Article: ] |
| 28. | Hillan K, Nordlinger B, Ballet F, Puts JP, Infante R. The healing of colonic anastomoses after early intraperitoneal chemotherapy: an experimental study in rats. J Surg Res. 1988;44:166-171. [Cited in This Article: ] |
| 29. | Sugarbaker PH, Gianola FJ, Speyer JC, Wesley R, Barofsky I, Meyers CE. Prospective, randomized trial of intravenous versus intraperitoneal 5-fluorouracil in patients with advanced primary colon or rectal cancer. Surgery. 1985;98:414-422. [Cited in This Article: ] |
| 30. | Scheithauer W, Kornek GV, Marczell A, Karner J, Salem G, Greiner R, Burger D, Stöger F, Ritschel J, Kovats E. Combined intravenous and intraperitoneal chemotherapy with fluorouracil + leucovorin vs fluorouracil + levamisole for adjuvant therapy of resected colon carcinoma. Br J Cancer. 1998;77:1349-1354. [Cited in This Article: ] |
| 31. | Kelsen DP, Saltz L, Cohen AM, Yao TJ, Enker W, Tong W, Tao Y, Bertino JR. A phase I trial of immediate postoperative intraperitoneal floxuridine and leucovorin plus systemic 5-fluorouracil and levamisole after resection of high risk colon cancer. Cancer. 1994;74:2224-2233. [Cited in This Article: ] |
| 32. | Graf W, Weiber S, Glimelius B, Jiborn H, Påhlman L, Zederfeldt B. Influence of 5-fluorouracil and folinic acid on colonic healing: an experimental study in the rat. Br J Surg. 1992;79:825-828. [Cited in This Article: ] |
| 33. | Kanellos I, Odisseos C, Zaraboukas T, Kavouni A, Galovatsea K, Dadoukis I. Colonic healing after early intraperitoneal administration of 5-fluorouracil and interferon in rats. Int J Colorectal Dis. 1997;12:45-48. [Cited in This Article: ] |
| 34. | Hananel N, Gordon PH. Effect of 5-fluorouracil and leucovorin on the integrity of colonic anastomoses in the rat. Dis Colon Rectum. 1995;38:886-890. [Cited in This Article: ] |
| 35. | Brown GL, Curtsinger LJ, White M, Mitchell RO, Pietsch J, Nordquist R, von Fraunhofer A, Schultz GS. Acceleration of tensile strength of incisions treated with EGF and TGF-beta. Ann Surg. 1988;208:788-794. [Cited in This Article: ] |
| 36. | Pinedo HM, Peters GF. Fluorouracil: biochemistry and pharmacology. J Clin Oncol. 1988;6:1653-1664. [Cited in This Article: ] |
| 37. | Kotake K, Koyama Y, Namba M, Sunagawa M, Ito M, Kadowaki A, Konishi F, Kanazawa K, Amemiya T, Akamatsu H. [Neoadjuvant chemotherapy with tegafur suppository for rectal cancer--effects of tegafur on nuclear DNA content of cancer cells--Tochigi Colorectal Cancer Study Group]. Gan To Kagaku Ryoho. 1995;22:793-798. [Cited in This Article: ] |
| 38. | Valeriote F, Santelli G. 5-Fluorouracil (FUra). Pharmacol Ther. 1984;24:107-132. [Cited in This Article: ] |
| 39. | Cohen SS, Flaks JG, Barner HD, Loeb MR, Lichtenstein J. The mode of action of 5-fluorouracil and its derivates. Proc Natl Acad Sci USA. 1958;44:1004-1012. [Cited in This Article: ] |
| 40. | Nyhlén A, Ljungberg B, Nilsson-Ehle I, Odenholt I. Postantibiotic effect of meropenem and ciprofloxacin in the presence of 5-fluorouracil. Chemotherapy. 2002;48:182-188. [Cited in This Article: ] |