Copyright
©2009 The WJG Press and Baishideng.
World J Gastroenterol. Aug 14, 2009; 15(30): 3713-3724
Published online Aug 14, 2009. doi: 10.3748/wjg.15.3713
Published online Aug 14, 2009. doi: 10.3748/wjg.15.3713
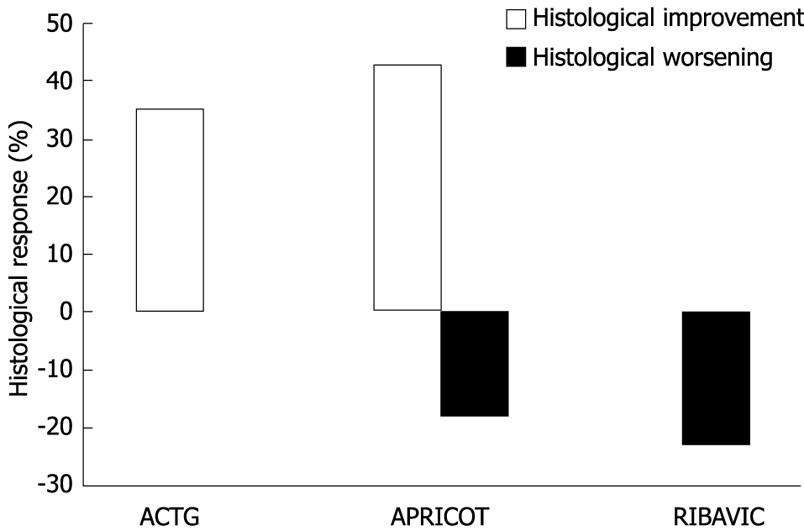
Figure 3 A beneficial histological response was seen in 35% and 43%, respectively, in patients with no sustained virologic response (SVR) in the ACTG and APRICOT trials.
Worsening histology was seen in 18% patients with no SVR in the APRICOT study; similar data were not available in the ACTG study. In the RIBAVIC study, histological worsening was seen in 23% without SVR while histology remained stable in the remaining patients.
- Citation: Singal AK, Anand BS. Management of hepatitis C virus infection in HIV/HCV co-infected patients: Clinical review. World J Gastroenterol 2009; 15(30): 3713-3724
- URL: https://www.wjgnet.com/1007-9327/full/v15/i30/3713.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.3713