Published online Aug 7, 2009. doi: 10.3748/wjg.15.3664
Revised: June 30, 2009
Accepted: July 7, 2009
Published online: August 7, 2009
AIM: To evaluate the efficacy of sequential use of transarterial chemoembolization (TACE) and percutaneous cryosurgery for unresectable hepatocellular carcinoma (HCC).
METHODS: Four hundred and twenty patients were enrolled in this study. The patients, who were considered to have unresectable tumors due to their location or size or comorbidity, were divided into sequential TACE-cryosurgery (sequential) group (n = 290) and cryosurgery alone (cryo-alone) group (n = 130). Patients in the sequential group tended to have larger tumors and a greater number of tumors than those in the cryo-alone group. Tumors larger than 10 cm in diameter were only seen in the sequential group. TACE was performed with the routine technique and percutaneous cryosurgery was conducted under the guidance of ultrasound 2-4 wk after TACE.
RESULTS: During a mean follow-up period of 42 ± 17 mo (range, 24-70 mo), the local recurrence rate at the ablated area was 17% for all patients, 11% and 23% for patients in sequential group and cryo-alone groups, respectively (P = 0.001). The overall 1-, 2-, 3-, 4- and 5-year survival rate was 72%, 57%, 47%, 39% and 31%, respectively. The 1- and 2-year survival rates (71% and 61%) in sequential group were similar to those (73% and 54%) in cryo-alone group (P = 0.69 and 0.147), while the 4- and 5-year survival rates were 49% and 39% in sequential group, higher than those (29% and 23%) in cryo-alone group (P = 0.001). Eighteen patients with large HCC (> 5 cm in diameter) survived for more than 5 years after sequential TACE while no patient with large HCC (> 5 cm in diameter) survived more than 5 years after cryosurgery. The overall complication rate was 24%, and the complication rates were 21% and 26% for the sequential and cryo-alone groups, respectively (P = 0.06). The incidence of hepatic bleeding was higher in cryo-alone group than in sequential group (P = 0.02). Liver crack only occurred in two patients of the cryo-alone group.
CONCLUSION: Pre-cryosurgical TACE can increase the cryoablation efficacy and decrease its adverse effects, especially bleeding. Sequential TACE and cryosurgery may be the better procedure for unresectable HCC, especially for large HCC.
- Citation: Xu KC, Niu LZ, Zhou Q, Hu YZ, Guo DH, Liu ZP, Lan B, Mu F, Li YF, Zuo JS. Sequential use of transarterial chemoembolization and percutaneous cryosurgery for hepatocellular carcinoma. World J Gastroenterol 2009; 15(29): 3664-3669
- URL: https://www.wjgnet.com/1007-9327/full/v15/i29/3664.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.3664
Cryosurgery has been used for two decades in treatment of many benign, malignant, and metastatic tumors[1–3]. Furthermore, hepatocellular carcinoma (HCC) has been successfully treated either with cryosurgery alone or in combination with resection[4–6].
Transarterial chemoembolization (TACE) itself is not associated with improved survival for patients with HCC[7]. However, it can shrink the HCC mass. It has been shown that sequential use of TACE and hepatectomy improves the outcome of patients with large HCC[8]. Cryosurgery is similar to the surgical resection of HCC in terms of total destruction of the tumor tissue. Therefore, TACE in combination with cryosurgery is expected to yield a better therapeutic effect on HCC, especially on large HCC.
From March 2001 to December 2006, 510 patients with HCC underwent percutaneous cryosurgery with or without TACE. In this study, we retrospectively investigated the results of 420 patients with HCC. Of them, 290 were treated with sequential use of TACE and percutaneous cryosurgery, and 130 received percutaneous cryosurgery alone.
A total of 420 patients (310 males and 110 females) at a median age of 43 years (range, 21-81 years) were enrolled in this study. The diagnosis of HCC was made by abdominal computed tomography (CT), liver ultrasonography, and/or whole positron emission tomography-CT (PET-CT). Two hundred and ninety-five patients had an elevated serum α-fetoprotein (AFP) level. HCC was proven by biopsy in 286 patients. All the patients were considered to have unresectable tumors due to their location or size or comorbidity. Patients with Child-Pugh C tumors and cirrhotic ascites were excluded from this study. The patients were divided into sequential TACE-cryosurgery (sequential) group (n = 290) and cryosurgery alone (cryo-alone) group (n = 130) (Table 1). There were no differences in age, sex and Child-Pugh classification of the patients between the two groups. However, patients in the sequential group tended to have larger tumors and a greater number of tumors compared to those in the cryo-alone group (P < 0.05). Tumors larger than 10 cm in diameter were only found in the sequential group. All patients gave their informed consent. The study was approved by the Ethical Committee of Fuda Cancer Hospital (Guangzhou, China).
| Clinical features | Sequential group (n = 290) | Cryo-alone group (n = 130) | P |
| Age (median) | 46 | 41 | |
| Sex (M/F) | 212/78 | 98/32 | 0.623 |
| Child-Pugh A | 91 (31.4) | 42 (32.3) | 0.850 |
| Child-Pugh B | 199 (68.6) | 88 (67.7) | 0.850 |
| Tumors | |||
| 1 | 132 (45.5) | 85 (57.7) | 0.001 |
| 2 | 84 (28.9) | 38 (25.4) | |
| 3 | 32 (11.0) | 6 (10.0) | |
| > 3 | 42 (14.5) | 1 ( 6.9) | |
| Largest diameter of tumors | |||
| Range (cm) | 4.5-15.0 | 3.1-7.0 | |
| mean ± SD (cm) | 6.5 ± 3.1 | 4.6 ± 3.2 | 0.04 |
| Tumor > 10 cm | 69 (23.8) | 0 | |
TACE was performed after cross-sectional images were reviewed as previously described[9]. A French vascular sheath was placed into the femoral artery, and a 0.035 inch diameter Mickaelson catheter was advanced into the celiac and superior mesenteric arteries. Contrast was injected into the arteries during rapid-sequence radiographic imaging. Arterial branches supplying the tumors were then located. The venous phase was carefully examined for patency of the portal veins. A 0.018 inch diameter Tracker catheter was advanced through the Mickaelson catheter to the arterial branches supplying the tumors. The mixture of doxorubicin (50 mg), mitomycin (10 mg), and lipiodol (4-15 mL) was injected into the arterial branches until hemostasis was achieved. If the tumors had no shrinkage 2 wk after the procedure, a second TACE was performed.
Percutaneous cryosurgery was performed under the guidance of ultrasound. Right lateral intercostal access was used to place cryoprobes in most patients. The size of cryoprobes used depended upon the tumor size and number. Small lesions were ablated with one or two 2-mm cryoprobes. Larger caliber cryoprobes (5-mm) or multiple probes were used according to the size of lesions. The cryoprobes were placed in the centre of tumor using a modified Seldinger technique under real-time ultrasound guidance. Multiple cryoprobes were were placed 1.5-2 cm apart in the tumor periphery. Once the cryoprobe was positioned, freezing was initiated using an argon/helium-based cryosurgery system (EndoCare, Inc., Irvine, Calif. USA). Two freeze cycles (15 min each) were applied for 10-15 min of helium thaw following each freeze. The size of the ice ball formed by the cryoprobes was evaluated by ultrasound and made at least 1 cm larger than the apparent ultrasonic size of the tumor.Generally, separate lesions were frozen sequentially rather than simultaneously. The probe was removed after the second freeze/thaw cycle and the tract was filled with Gelfoam strips soaked in thrombin to facilitate hemostasis[1011].
Follow-up consisted of ultrasonography and/or helical CT of the liver and serial check of AFP levels 1 mo after surgery and then every 2-3 mo for 1-2 years. Eighty-two patients underwent PET-CT during the follow-up and were subsequently reassessed every 6 mo with PET-CT. Successful tumor ablation (disease-free) was ideally defined by disappearance or no expansion of the ablated lesion on CT follow-up or no metabolic activity on PET-CT. Tumor recurrence or persistence was also defined using CT. Percutaneous biopsy was used in 79 patients to verify the eradication or recurrence of tumors.
Survival rate of the patients was calculated from the time of cryosurgery to the time of death or last follow-up with Kaplan-Meier method (J Am Stat Assoc 1958; 53: 457-481). Values were expressed as mean ± SD. Variables were compared using Fisher’s exact test (two-sided) or rank-sum test when appropriate. P < 0.05 was considered statistically significant. SPSS 9.0 (SPSS, Chicago, Illinois) was used for data analysis.
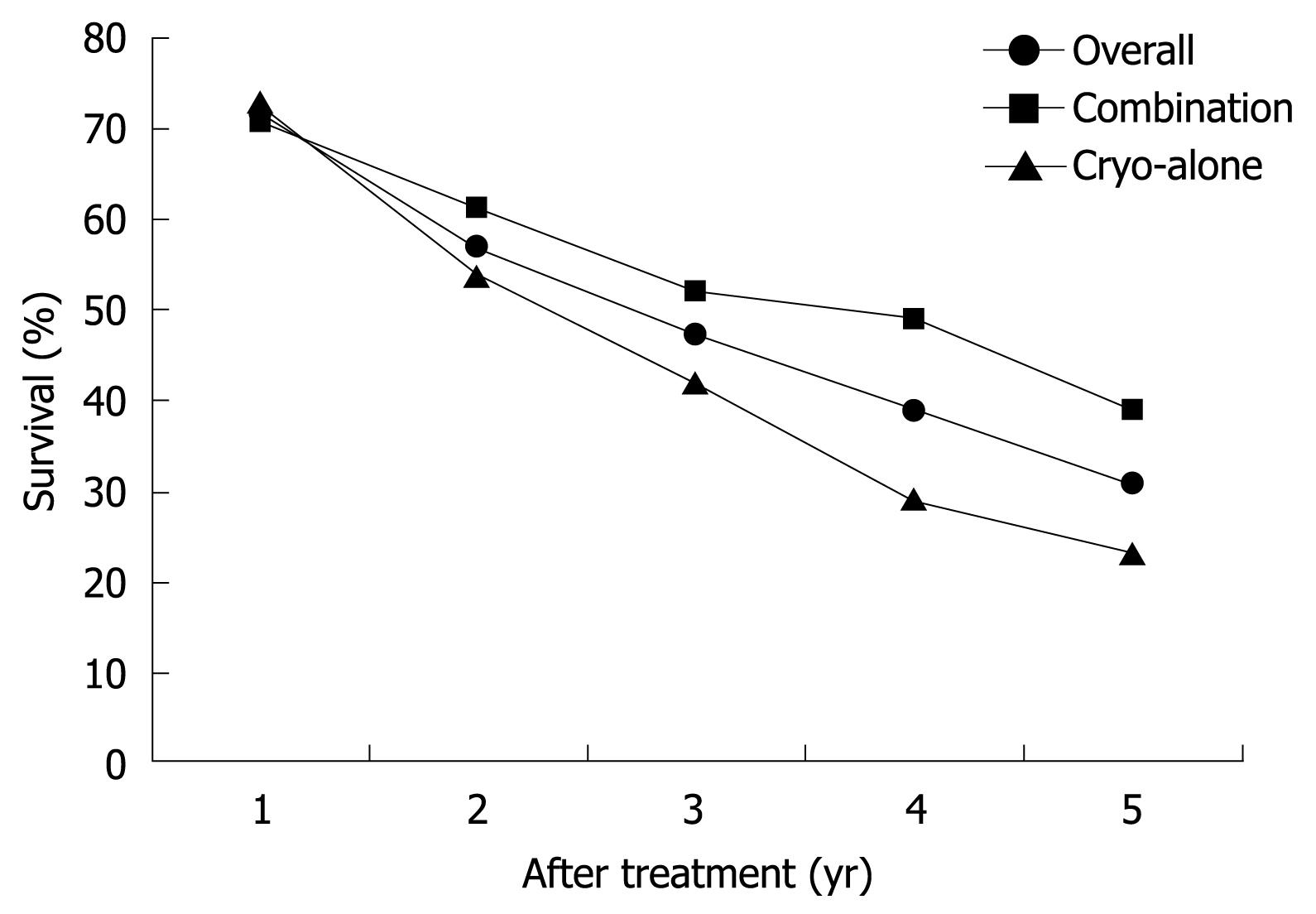
The patients were followed up for 42 ± 17 mo (range, 24-70 mo) (Table 2). The local recurrence rate at the ablated area was 17% for all patients, and 11% and 23% for sequential and cryo-alone groups, respectively (P = 0.001). The overall 1-, 2-, 3-, 4- and 5-year survival rate was 72%, 57%, 47%, 39% and 31%, respectively. The 1- and 2-year survival rates (71% and 61%) for sequential group were similar to those (73% and 54%) for cryo-alone group (P = 0.69 and 0.147, respectively), while the 4- and 5-year survival rates were 49% and 39% for sequential group, higher than those (29% and 23%) for cryo-alone group (P = 0.001, Figure 1).
| Patients (n = 420) | Sequential group (n = 290) | Cryo-alone group (n = 130) | P | |
| Local recurrence | 17 | 11 | 24 | 0.001 |
| Survival rate | ||||
| 1-yr | 72 | 71 | 73 | 0.668 |
| 2-yr | 57 | 61 | 54 | 0.147 |
| 3-yr | 47 | 52 | 42 | 0.064 |
| 4-yr | 39 | 49 | 29 | 0.001 |
| 5-yr | 31 | 39 | 23 | 0.001 |
Eighteen patients with large HCC (> 5 cm in diameter) survived more than 5 years after sequential TACE-cryosurgery while no patient with large HCC survived more than 5 years after cryosurgery alone.
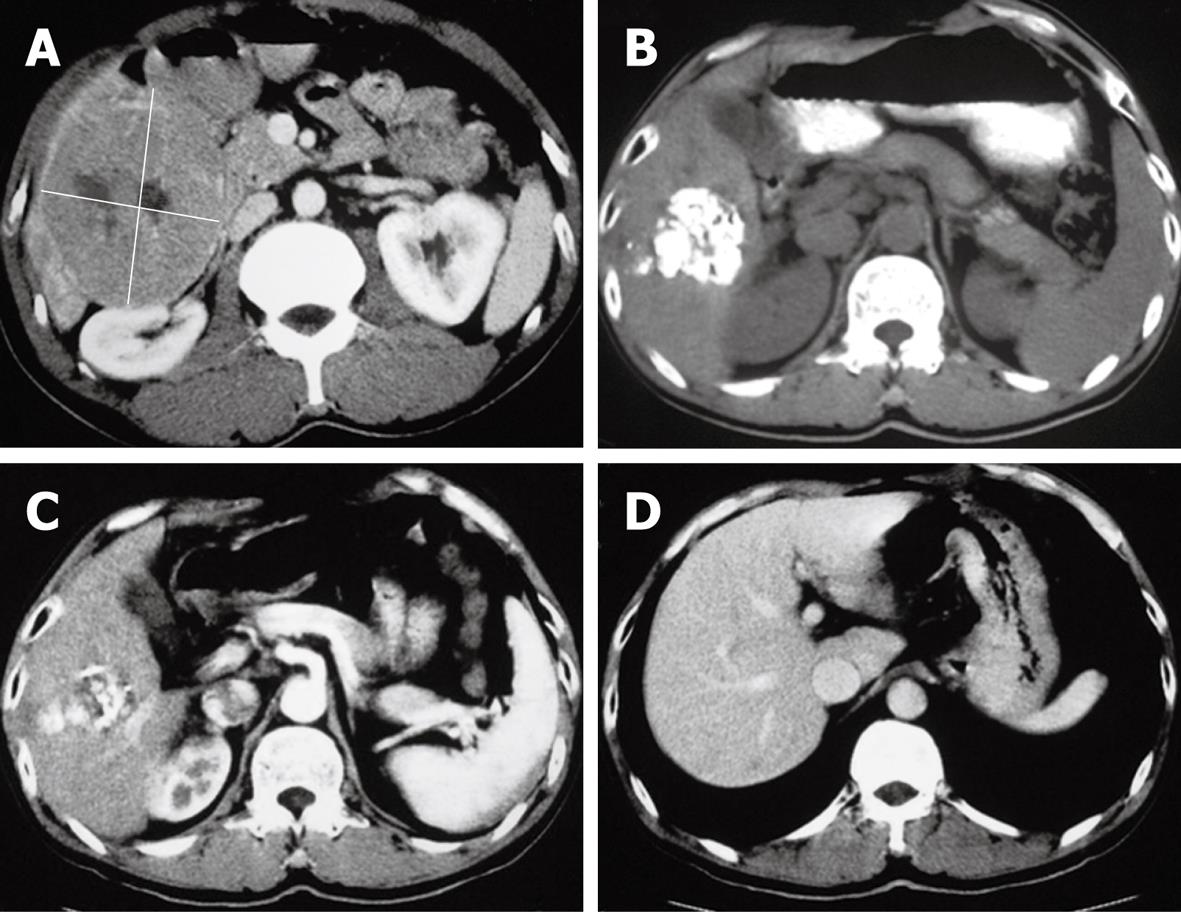
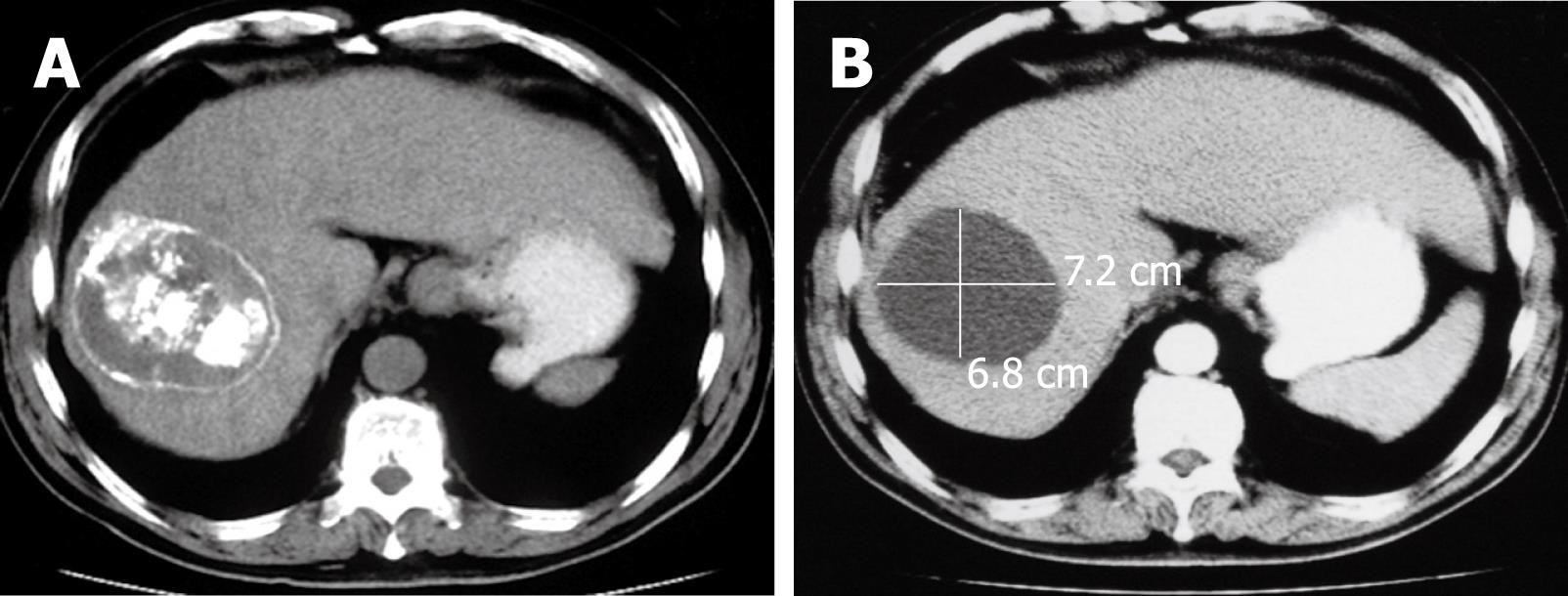
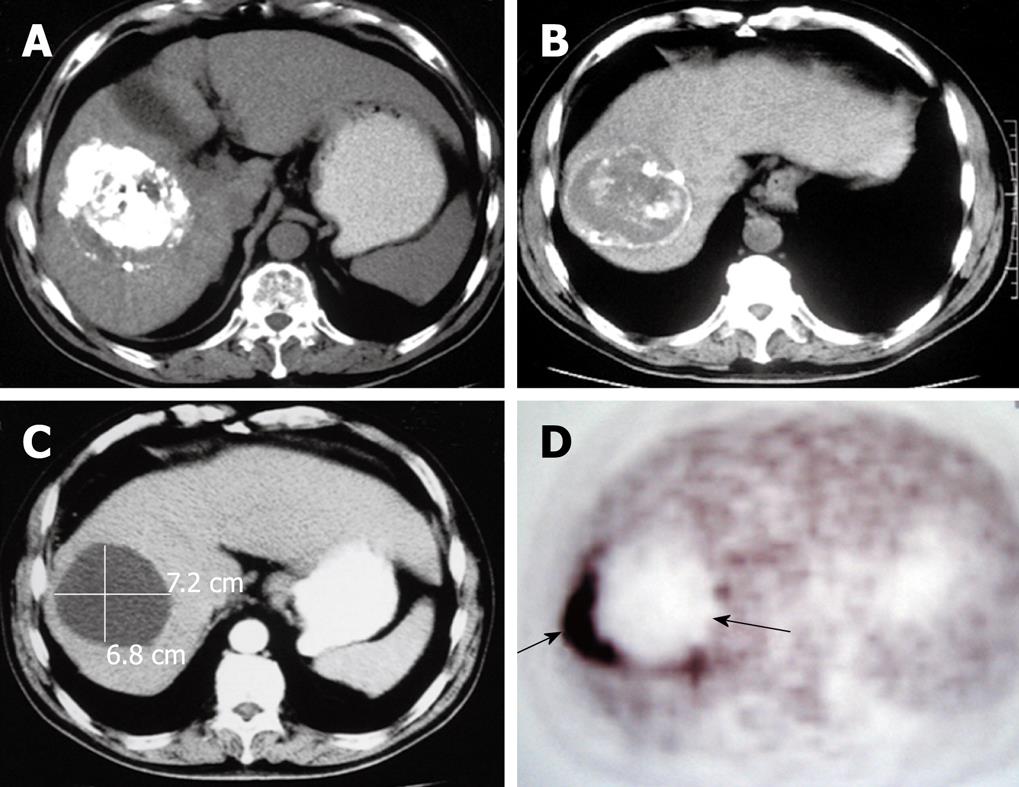
The CT of liver for selected patients is illustrated in Figures 2, 3 and 4.
The main complications were hepatic bleeding, liver crack and failure, thrombocytopenia and/or clotting dysfunction, acute renal failure with myoglobinuria and pneumonia (Table 3). The overall complication rate was 24%, and the complication rate was 21% and 26%, respectively, for the sequential and cryo-alone groups (P = 0.184). The incidence of hepatic bleeding was higher in cryo-alone group than in sequential group (P = 0.001). Liver crack only occurred in the cryo-alone group. The incidence of thrombocytopenia and/or clotting dysfunction, liver failure and pneumonia was similar in the two groups (P = 0.599). Acute renal failure with myoglobinuria was observed in two patients of the sequential group and in one patient of the cryo-alone group. Agranulocytosis occurred in only four patients of the sequential group. Two patients in the cryo-alone group died of liver crack and massive hepatic bleeding during peri-cryosurgery.
| Patients (n = 420) | TACE-cryosurgery (n = 290) | Cryo-alone (n = 130) | P | |
| Hepatic bleeding | 16 (3.8) | 5 (1.7) | 11 (8.5) | 0.001 |
| Liver crack | 2 (0.5) | 0 | 2 (1.5) | |
| Thrombocytopenia and/or clotting dysfunction | 30 (7.1) | 21 (7.2) | 9 (6.9) | 0.907 |
| Liver failure | 16 (3.8) | 12 (4.1) | 4 (3.1) | 0.599 |
| Acute renal failure with myoglobinuria | 3 (0.7) | 2 (0.7) | 1 (0.7) | |
| Agranulocytosis | 4 (0.9) | 4 (1.4) | 0 | |
| Pneumonia | 25 (0.6) | 17 (5.9) | 8 (6.2) | 0.907 |
| Total | 101 (24.0) | 61 (21.0) | 40 (30.7) | 0.184 |
Surgical resection (hepatectomy) can achieve a long-term survival time in up to 30% of patients with HCC. Unfortunately, because of the limitations of surgical techniques and patient condition, only 25% or less of patients are estimated to be candidates for resection of HCC[12]. Since cryosurgery is a focal treatment, sparing more normal liver tissues than resection, it can be used for unresectable multiple lesions affecting both liver lobes and large tumors abutting major vessels[1314]. It has been shown that mini-invasively percutaneous hepatic cryosurgery under guidance of real time ultrasound can achieve a long-term survival of patients with both primary and metastatic hepatic malignancies[1516].
Cryoablation is effective for tumor mass, but postcryosurgical recurrence is often observed due to insufficient freezing during cryosurgery[17]. Overcoming this problem is the key to the improvement of overall survival rate of patients.
TACE is used either as an adjuvant therapy or as a palliative procedure for patients with unresectable HCC[1819]. It has been shown that TACE prior to surgical resection can not only prevent recurrence of HCC by controlling intrahepatic spread via the portal system, but also facilitate surgery by reducing tumor bulk[20]. Cryosurgery, in which tumors are frozen and then left in situ to be reabsorbed, is equivalent to the surgical resection of HCC in terms of total destruction of the tumor tissue, indicating that it is reasonable to perform TACE for tumors prior to cryosurgery.
In our study, the 1-, 2-, 3, 4- and 5-year survival rate was 71%, 61%, 52%, 49% and 39%, respectively, for the 290 patients after sequential TACE-cryosurgery, and 73%, 54%, 42%, 29% and 23%, respectively, for the 130 patients after cryo-alone. The 4- and 5-year survival rates were higher for the patients of sequential group than for those of cryo-alone group, suggesting that cryosurgery in combination with TACE can achieve a better long-term outcome, which is consistent with the reported findings[21].
The relatively good outcome obtained in our study was due to the complementary effect of sequential TACE and cryosurgery.
Several factors can influence cryo-induced tumor cell destruction. Among these factors, temperature is a critical factor since a temperature lower than -40°C is assumed necessary to ensure tumor ablation. An ice-ball larger than the target lesion is thus necessary for complete tumor ablation, because the temperature at the edge (a few millimeters in thickness) of an ice-ball is non-lethal[22]. An ice-ball encompassing the entire tumor mass 1 cm beyond the tumor border should be considered adequate for ablation. According to the MRI-estimated three-dimensional temperature distribution in liver cryo-lesions[23], the mean value of distances calculated between the -40°C isotherm and cryolesion edge is a median of 4.1 mm and the largest distance defined for each cryo-lesion is a median of 8.1 mm. However, the distance is related to the volume of the cryo-treated tumor. The largest distance defined between the the -40°C isotherm and the edge of cryo-lesion may be close to 1 cm for a cryo-lesion volume less than 25 cm3, while a cryo-lesion larger than 25 cm3, the 1-cm ice-ball extension cannot ensure the temperature adequate for ablation in the entire volume enclosed by the rim zone. It has been shown that tumor size influences the tumor ablation rate, and lesions larger than 3 cm are more likely associated with a higher recurrence rate than smaller tumors[24].
Therefore, the smaller the tumor is, the more effective the cryoablation is and the lower the recurrence rate is. Since TACE can shrink HCC mass, it can be expected to increase the efficacy of cryoablation for HCC, and to decrease its local recurrence if performed prior to cryoablation. In our study, the recurrence rate of HCC was 11% for the sequential group and 23% for the cryo-alone group, respectively, that is of special significance for large tumors close to large vessels, because the temperature cannot reach the edge zone of cryo-lesions due to its blood warming effect[2526]. This opinion is supported by our study in which 18 patients with large HCC (> 5 cm in diameter) survived more than 5 years after sequential TACE while no patient with large HCC survived more than 5 years after cryosurgery alone.
The major complications of cryosurgery are hemorrhage and liver crack[2728]. In our study, post-cryosurgery hepatic bleeding occurred in 16 patients. Of them, 11 (8.5%) received cryo-alone and 5 (1.7%) underwent sequential TACE. The most dangerous liver crack was observed in only two patients of the cryo-alone group. Clavien et al[21] treated 15 HCC patients with cryosurgery and found that only one patient who did not undergo pre-cryosurgery TACE had hepatic bleeding, suggesting that TACE can reduce post-cryosurgery complications, such as bleeding and liver crack.
Pre-surgical TACE may be contraindicated in patients with resectable HCC and cirrhosis, because it causes progressive deterioration of liver function[29]. However, Kaibori et al[20] reported that TACE prior to HCC resection improves the prognosis of HCC patients with severe liver dysfunction, because decreasing resection of non-tumorous liver tissue avoids postoperative hepatic failure in patients with cirrhosis. In our study, liver failure occurred in 16 patients (3.8%), including 12 patients in the sequential group and four patients in the cryo-alone group, with no increased risk of liver failure caused by TACE. Other adverse effects of cryosurgery, such as thrombocytopenia and/or clotting dysfunction, acute renal failure with myoglobinuria, were observed in both groups of patients. In our study, four patients in the sequential group developed agranulocytosis due to a higher dose of chemotherapeutic agents used in TACE. The complication became less when the dose of chemotherapeutic agents was reduced.
In conclusion, TACE prior to cryosurgery can increase cryoablation efficacy and decrease its adverse effects, especially bleeding. Sequential use of TACE and cryosurgery may be a better procedure for improving the outcome of patients with unresectable HCC, especially large HCC. Further prospective study is required to fully evaluate its value.
Hepatocellular carcinoma (HCC) is one of the most common cancers in the world. Its treatment modalities, including surgical resection or liver transplantation, benefit only a small proportion of such patients. Cryosurgery, one of the proposed therapies for HCC, can improve the survival time of such patients.
Although cryosurgery is considered an effective treatment modality for unresectable HCC, it has been performed only for smaller tumors. Selected patients with stage III/IV HCC can be down staged with transarterial chemoembolization (TACE). Importantly, patients who are successfully down staged have a higher overall survival rate rafter resection of HCC. TACE prior to cryosurgery should be a good choice for HCC.
Few reports are available on combined TACE and percutaneous cryosurgery. This study showed that pre-cryosurgical TACE could increase the cryoablation efficacy and decrease its adverse effects, especially bleeding, and improve the outcome of patients with unresectable HCC, especially large HCC.
To the best of our knowledge, this is the first study comparing sequential TACE and cryosurgery for HCC patients. The results of this study show that sequential TACE and cryosurgery are a rather good treatment modality for HCC.
Cryosurgery, also known as cryotherapy or cryoablation, has gone through a long process of development. From the end of the 20th century, the development of imaging techniques and new freezing equipments has led to the emergence of modern cryosurgery. Liquid nitrogen operation system and argon-helium surgical system are representative of two important stages of modern cryosurgery. Apart from liver cancer, cryosurgery has been successfully performed for a variety of tumors.
The study seems to be very interesting. The results, based on clinical observation and follow-up, suggest that sequential TACE and percutaneous cryosurgery are a better choice for unresectable HCC, especially large HCC.
| 1. | Gage AA, Baust JG. Cryosurgery for tumors. J Am Coll Surg. 2007;205:342-356. [Cited in This Article: ] |
| 2. | Gage AA, Baust JG. Cryosurgery - a review of recent advances and current issues. Cryo Letters. 2002;23:69-78. [Cited in This Article: ] |
| 3. | Callstrom MR, Charboneau JW. Technologies for ablation of hepatocellular carcinoma. Gastroenterology. 2008;134:1831-1835. [Cited in This Article: ] |
| 4. | Crews KA, Kuhn JA, McCarty TM, Fisher TL, Goldstein RM, Preskitt JT. Cryosurgical ablation of hepatic tumors. Am J Surg. 1997;174:614-617; discussion 617-618. [Cited in This Article: ] |
| 5. | Lam CM, Yuen WK, Fan ST. Hepatic cryosurgery for recurrent hepatocellular carcinoma after hepatectomy: a preliminary report. J Surg Oncol. 1998;68:104-106. [Cited in This Article: ] |
| 6. | Sheen AJ, Poston GJ, Sherlock DJ. Cryotherapeutic ablation of liver tumours. Br J Surg. 2002;89:1396-1401. [Cited in This Article: ] |
| 7. | Lee KT, Lu YW, Wang SN, Chen HY, Chuang SC, Chang WT, Shi HY, Ker CG, Chiu HC. The effect of preoperative transarterial chemoembolization of resectable hepatocellular carcinoma on clinical and economic outcomes. J Surg Oncol. 2009;99:343-350. [Cited in This Article: ] |
| 8. | Yu YQ, Xu DB, Zhou XD, Lu JZ, Tang ZY, Mack P. Experience with liver resection after hepatic arterial chemoembolization for hepatocellular carcinoma. Cancer. 1993;71:62-65. [Cited in This Article: ] |
| 9. | Liaw YF, Lin DY. Transcatheter hepatic arterial embolization in the treatment of hepatocellular carcinoma. Hepatogastroenterology. 1990;37:484-488. [Cited in This Article: ] |
| 10. | Wong WS, Patel SC, Cruz FS, Gala KV, Turner AF. Cryosurgery as a treatment for advanced stage hepatocellular carcinoma: results, complications, and alcohol ablation. Cancer. 1998;82:1268-1278. [Cited in This Article: ] |
| 11. | Brewer WH, Austin RS, Capps GW, Neifeld JP. Intraoperative monitoring and postoperative imaging of hepatic cryosurgery. Semin Surg Oncol. 1998;14:129-155. [Cited in This Article: ] |
| 12. | Song TJ, Ip EW, Fong Y. Hepatocellular carcinoma: current surgical management. Gastroenterology. 2004;127:S248-S260. [Cited in This Article: ] |
| 13. | Neeleman N, Wobbes T, Jager GJ, Ruers TJ. Cryosurgery as treatment modality for colorectal liver metastases. Hepatogastroenterology. 2001;48:325-329. [Cited in This Article: ] |
| 14. | Goering JD, Mahvi DM, Niederhuber JE, Chicks D, Rikkers LF. Cryoablation and liver resection for noncolorectal liver metastases. Am J Surg. 2002;183:384-389. [Cited in This Article: ] |
| 15. | Xu KC, Niu LZ, He WB, Guo ZQ, Hu YZ, Zuo JS. Percutaneous cryoablation in combination with ethanol injection for unresectable hepatocellular carcinoma. World J Gastroenterol. 2003;9:2686-2689. [Cited in This Article: ] |
| 16. | Xu KC, Niu LZ, He WB, Hu YZ, Zuo JS. Percutaneous cryosurgery for the treatment of hepatic colorectal metastases. World J Gastroenterol. 2008;14:1430-1436. [Cited in This Article: ] |
| 17. | Baust J, Gage AA, Ma H, Zhang CM. Minimally invasive cryosurgery--technological advances. Cryobiology. 1997;34:373-384. [Cited in This Article: ] |
| 18. | Yamada R, Sato M, Kawabata M, Nakatsuka H, Nakamura K, Takashima S. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology. 1983;148:397-401. [Cited in This Article: ] |
| 19. | Yamada R, Kishi K, Sato M, Sonomura T, Nishida N, Tanaka K, Shioyama Y, Terada M, Kimura M. Transcatheter arterial chemoembolization (TACE) in the treatment of unresectable liver cancer. World J Surg. 1995;19:795-800. [Cited in This Article: ] |
| 20. | Kaibori M, Matsui Y, Kitade H, Kwon AH, Kamiyama Y. Hepatic resection for hepatocellular carcinoma in severely cirrhotic livers. Hepatogastroenterology. 2003;50:491-496. [Cited in This Article: ] |
| 21. | Clavien PA, Kang KJ, Selzner N, Morse MA, Suhocki PV. Cryosurgery after chemoembolization for hepatocellular carcinoma in patients with cirrhosis. J Gastrointest Surg. 2002;6:95-101. [Cited in This Article: ] |
| 22. | Popken F, Seifert JK, Engelmann R, Dutkowski P, Nassir F, Junginger T. Comparison of iceball diameter and temperature distribution achieved with 3-mm accuprobe cryoprobes in porcine and human liver tissue and human colorectal liver metastases in vitro. Cryobiology. 2000;40:302-310. [Cited in This Article: ] |
| 23. | Mala T, Samset E, Aurdal L, Gladhaug I, Edwin B, Søreide O. Magnetic resonance imaging-estimated three-dimensional temperature distribution in liver cryolesions: a study of cryolesion characteristics assumed necessary for tumor ablation. Cryobiology. 2001;43:268-275. [Cited in This Article: ] |
| 24. | Seifert JK, Gerharz CD, Mattes F, Nassir F, Fachinger K, Beil C, Junginger T. A pig model of hepatic cryotherapy. In vivo temperature distribution during freezing and histopathological changes. Cryobiology. 2003;47:214-226. [Cited in This Article: ] |
| 25. | Seifert JK, Junginger T. Prognostic factors for cryotherapy of colorectal liver metastases. Eur J Surg Oncol. 2004;30:34-40. [Cited in This Article: ] |
| 26. | Pearson AS, Izzo F, Fleming RY, Ellis LM, Delrio P, Roh MS, Granchi J, Curley SA. Intraoperative radiofrequency ablation or cryoablation for hepatic malignancies. Am J Surg. 1999;178:592-599. [Cited in This Article: ] |
| 27. | Seifert JK, Morris DL. World survey on the complications of hepatic and prostate cryotherapy. World J Surg. 1999;23:109-113; discussion 113-114. [Cited in This Article: ] |
| 28. | Sarantou T, Bilchik A, Ramming KP. Complications of hepatic cryosurgery. Semin Surg Oncol. 1998;14:156-162. [Cited in This Article: ] |
| 29. | Uchida M, Kohno H, Kubota H, Hayashi T, Yamanoi A, Kimoto T, Ono T, Nagasue N. Role of preoperative transcatheter arterial oily chemoembolization for resectable hepatocellular carcinoma. World J Surg. 1996;20:326-331. [Cited in This Article: ] |