Copyright
©2009 The WJG Press and Baishideng.
World J Gastroenterol. Mar 28, 2009; 15(12): 1431-1442
Published online Mar 28, 2009. doi: 10.3748/wjg.15.1431
Published online Mar 28, 2009. doi: 10.3748/wjg.15.1431
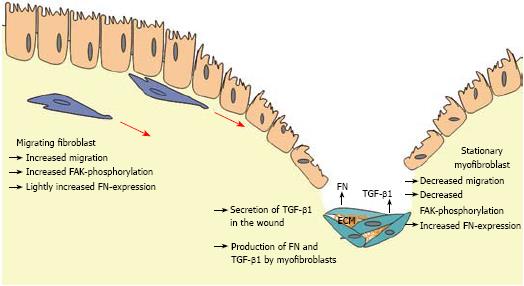
Figure 9 Schematic illustration of the role of TGF-β1, FN, and FAK in the development of fibrosis in CD.
After wounding TGF-β1 is produced by different cell types and connective tissue cells. CLPF increase FAK phosphorylation and migrate into the wound along the TGF-β1 gradient. FN that is also produced during migration induction and secreted at the site of injury may lead to an additional enhancement of the migratory gradient. Long term contact with TGF-β1 within the wound allows fibroblasts to differentiate into myofibroblasts. These cells have a reduced migratory potential and support wound healing via wound contraction and production of extracellular matrix deposition like FN. Phosphorylation of FAK is reduced after longer contact with TGF-β1 due to the differentiation into ECM-producing myofibroblasts.
- Citation: Brenmoehl J, Miller SN, Hofmann C, Vogl D, Falk W, Schölmerich J, Rogler G. Transforming growth factor-β1 induces intestinal myofibroblast differentiation and modulates their migration. World J Gastroenterol 2009; 15(12): 1431-1442
- URL: https://www.wjgnet.com/1007-9327/full/v15/i12/1431.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.1431