Copyright
©2008 The WJG Press and Baishideng.
World J Gastroenterol. Dec 14, 2008; 14(46): 7059-7067
Published online Dec 14, 2008. doi: 10.3748/wjg.14.7059
Published online Dec 14, 2008. doi: 10.3748/wjg.14.7059
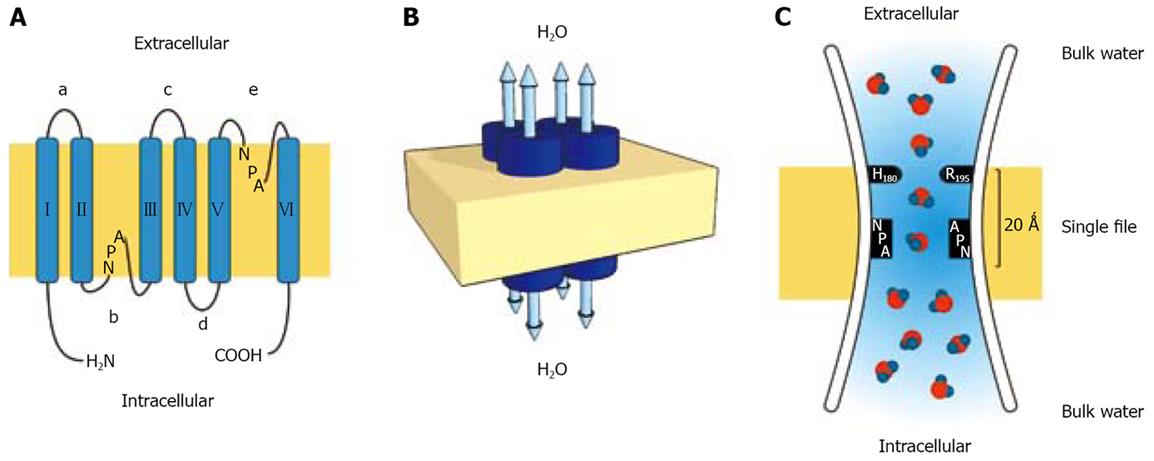
Figure 1 Topology, organization and functioning of the aquaporin water channel molecule.
A: Each AQP monomer consist of six transmembrane domains (I-VI) connected by five loops (a-e) with two NPA boxes shaping the water pore, and the amino and carboxy termini oriented toward the cytoplasm; B: Aquaporins are arranged in tetramers. The water pore does not reside in the center of the molecule, but is formed by connecting loops b and e in each subunit that functions as a unique water pore allowing bidirectional water movement; C: The hourglass model for aquaporin structure. The channel consist of an extracellular and intracellular vestibule containing water in bulk solution joined in the center by a central constriction 20 Å in length where water molecules pass in single file. The a/R constriction delimited by arginine in the position 195 (R195) and histidine in the position 180 (H180) provides fixed positive charges which prevent proton passage. The second constriction is bounded by two asparagine residues from the highly conserved NPA motif. The single water molecule passes through the constriction with no resistance as it forms transient hydrogen bonds with the nearby asparagines.
- Citation: Lehmann GL, Larocca MC, Soria LR, Marinelli RA. Aquaporins: Their role in cholestatic liver disease. World J Gastroenterol 2008; 14(46): 7059-7067
- URL: https://www.wjgnet.com/1007-9327/full/v14/i46/7059.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.7059